Tera Watts Tera Grams Tera Liters Workshop on





- Slides: 33

Tera. Watts, Tera. Grams, Tera. Liters Workshop on Challenges and Opportunities for Sustainable Production of Chemicals and Fuels beyond the Shale Gale Solar Energy-driven Photoelectrochemical Conversion Using Earth-Abundant Materials Gengfeng Zheng Fudan University 2015 -02 -02, UCSB

Acknowledgements Collaborators: Dongyuan Zhao (Fudan, Chemistry) Wenbin Cai (Fudan, Chemistry) Xingao Gong (Fudan, Physics) Min Jiang (Fudan, Life Sciences) Zhongqin Yang (Fudan, Physics) Song Jin (U. Wisconsin, Chemistry) Rong Fan (Yale, Bio. Med. Eng) Ahmed Elzatahry (King Saud Univ. ) Students: Jing Tang (Ph. D) Biao Kong (Ph. D) Yongcheng Wang (MS) Peimei Da (Ph. D) Yuhang Wang (Ph. D) 2

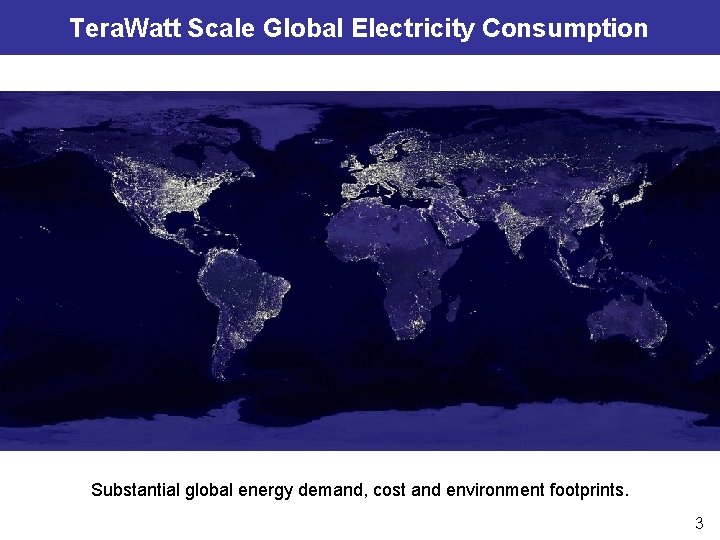
Tera. Watt Scale Global Electricity Consumption Substantial global energy demand, cost and environment footprints. 3

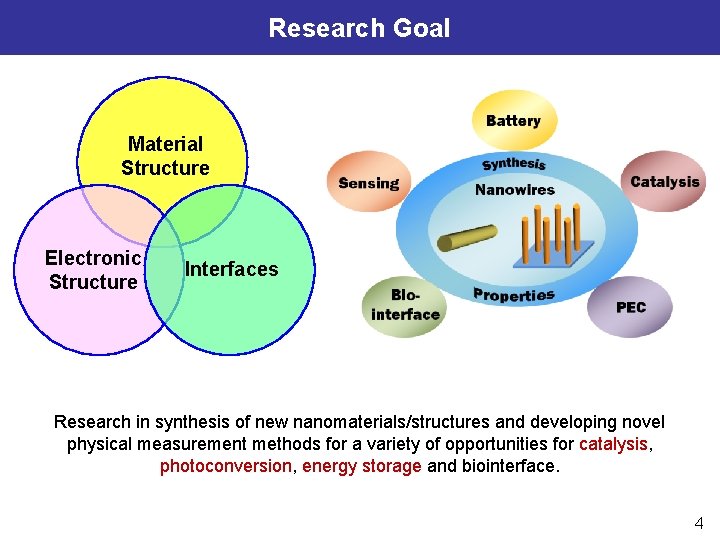
Research Goal Material Structure Electronic Structure Interfaces Research in synthesis of new nanomaterials/structures and developing novel physical measurement methods for a variety of opportunities for catalysis, photoconversion, energy storage and biointerface. 4

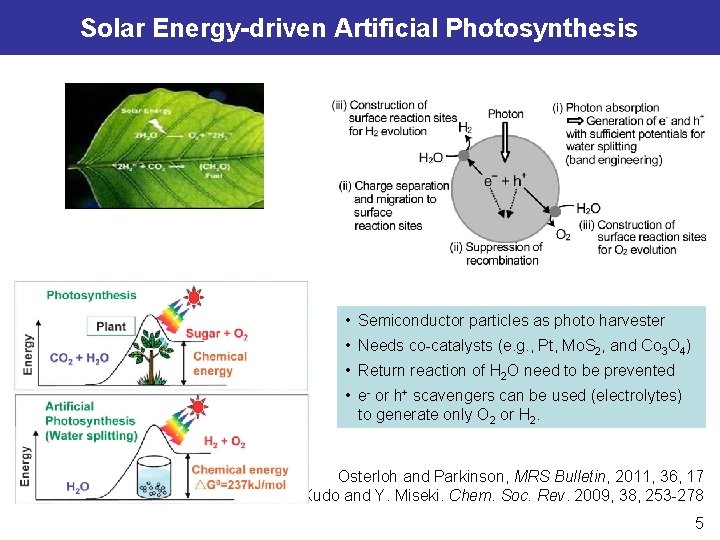
Solar Energy-driven Artificial Photosynthesis • Semiconductor particles as photo harvester • Needs co-catalysts (e. g. , Pt, Mo. S 2, and Co 3 O 4) • Return reaction of H 2 O need to be prevented • e- or h+ scavengers can be used (electrolytes) to generate only O 2 or H 2. Osterloh and Parkinson, MRS Bulletin, 2011, 36, 17 A. Kudo and Y. Miseki. Chem. Soc. Rev. 2009, 38, 253 -278 5

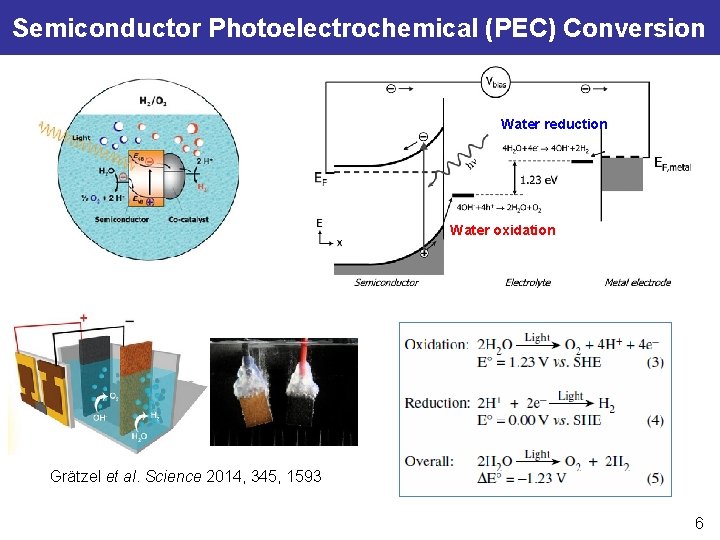
Semiconductor Photoelectrochemical (PEC) Conversion Water reduction Water oxidation Grätzel et al. Science 2014, 345, 1593 6

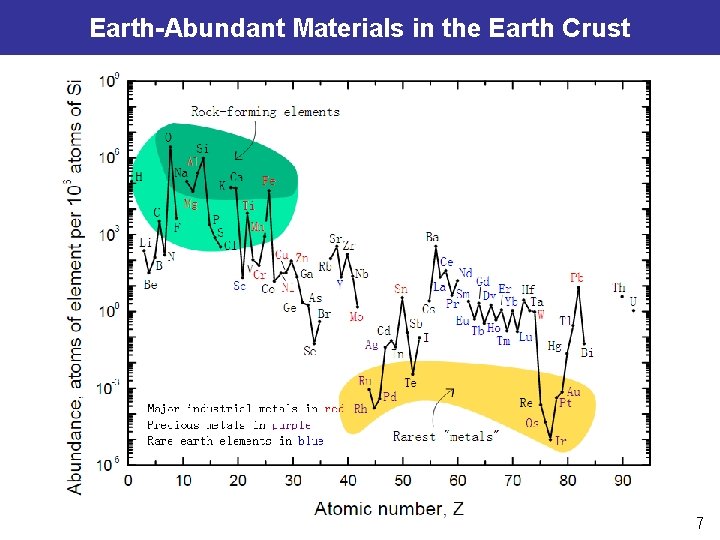
Earth-Abundant Materials in the Earth Crust 7

Sustainable Solar Energy Conversion 8

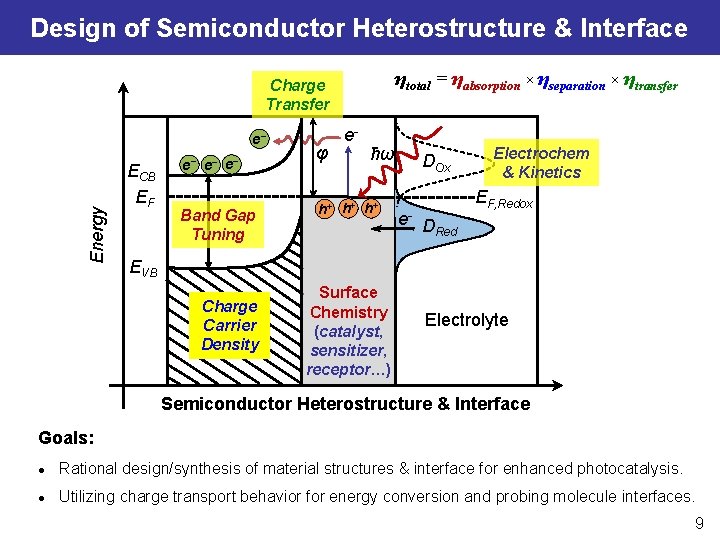
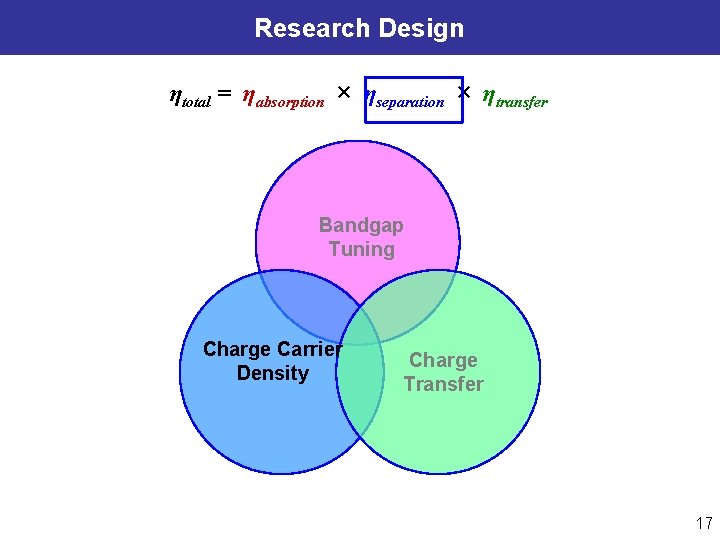
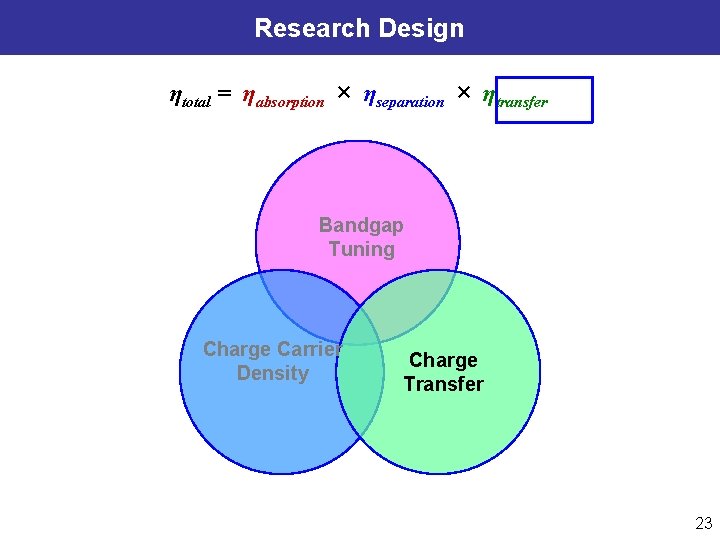
Design of Semiconductor Heterostructure & Interface ηtotal = ηabsorption × ηseparation × ηtransfer Charge Transfer Energy e− ECB EF e− e− e− Band Gap Tuning φ e- ħω h+ h+ h+ DOx e- D Red Electrochem & Kinetics EF, Redox EVB Charge Carrier Density Surface Chemistry (catalyst, sensitizer, receptor…) Electrolyte Semiconductor Heterostructure & Interface Goals: l Rational design/synthesis of material structures & interface for enhanced photocatalysis. l Utilizing charge transport behavior for energy conversion and probing molecule interfaces. 9

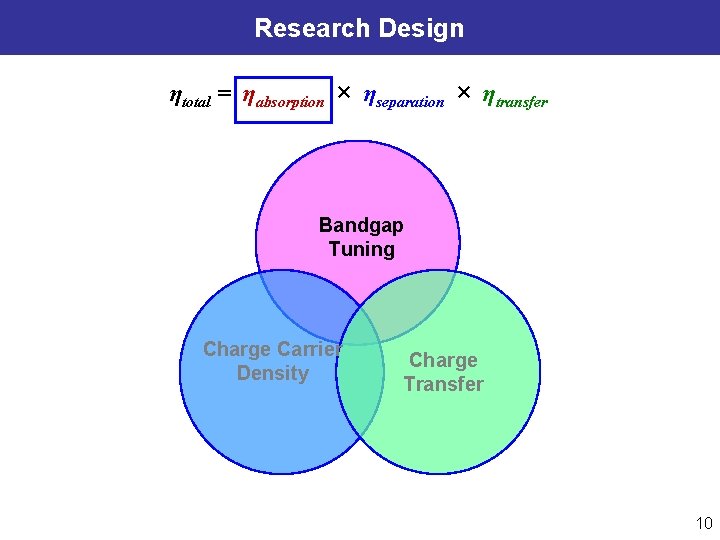
Research Design ηtotal = ηabsorption × ηseparation × ηtransfer Bandgap Tuning Charge Carrier Density Charge Transfer 10

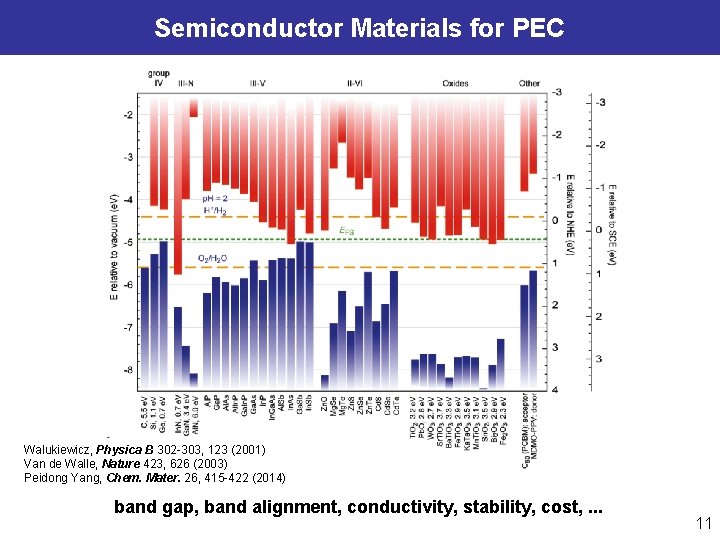
Semiconductor Materials for PEC Walukiewicz, Physica B 302 -303, 123 (2001) Van de Walle, Nature 423, 626 (2003) Peidong Yang, Chem. Mater. 26, 415 -422 (2014) band gap, band alignment, conductivity, stability, cost, . . . 11

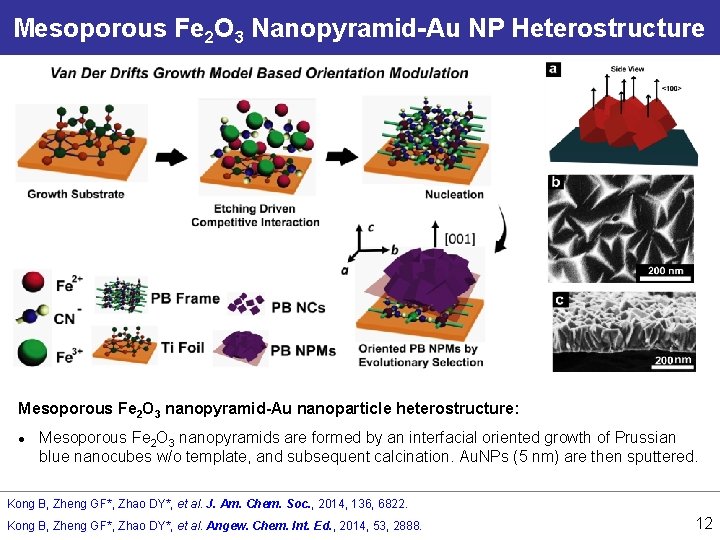
Mesoporous Fe 2 O 3 Nanopyramid-Au NP Heterostructure Mesoporous Fe 2 O 3 nanopyramid-Au nanoparticle heterostructure: l Mesoporous Fe 2 O 3 nanopyramids are formed by an interfacial oriented growth of Prussian blue nanocubes w/o template, and subsequent calcination. Au. NPs (5 nm) are then sputtered. Kong B, Zheng GF*, Zhao DY*, et al. J. Am. Chem. Soc. , 2014, 136, 6822. Kong B, Zheng GF*, Zhao DY*, et al. Angew. Chem. Int. Ed. , 2014, 53, 2888. 12

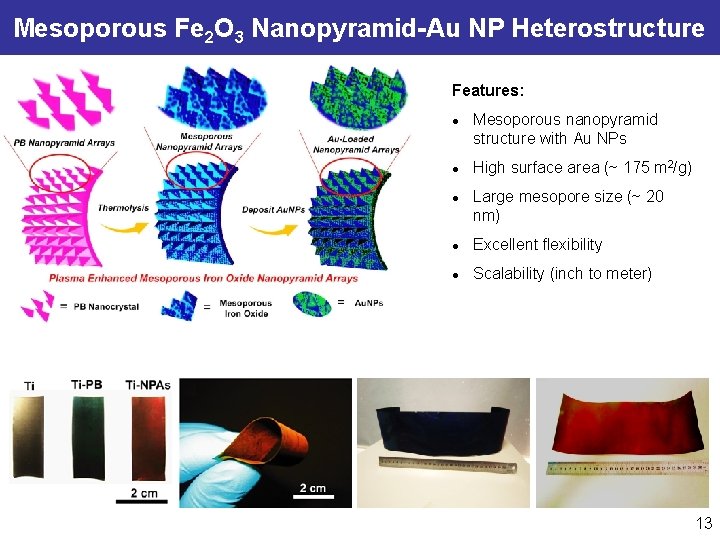
Mesoporous Fe 2 O 3 Nanopyramid-Au NP Heterostructure Features: l l l Mesoporous nanopyramid structure with Au NPs High surface area (~ 175 m 2/g) Large mesopore size (~ 20 nm) l Excellent flexibility l Scalability (inch to meter) 13

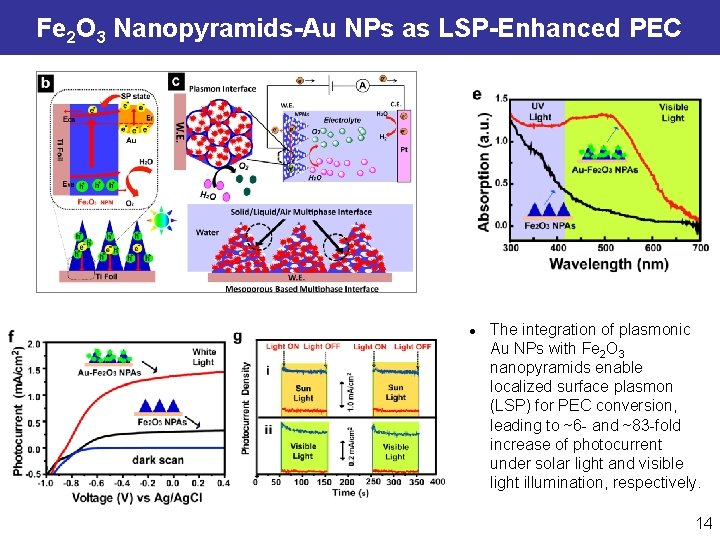
Fe 2 O 3 Nanopyramids-Au NPs as LSP-Enhanced PEC l The integration of plasmonic Au NPs with Fe 2 O 3 nanopyramids enable localized surface plasmon (LSP) for PEC conversion, leading to ~6 - and ~83 -fold increase of photocurrent under solar light and visible light illumination, respectively. 14

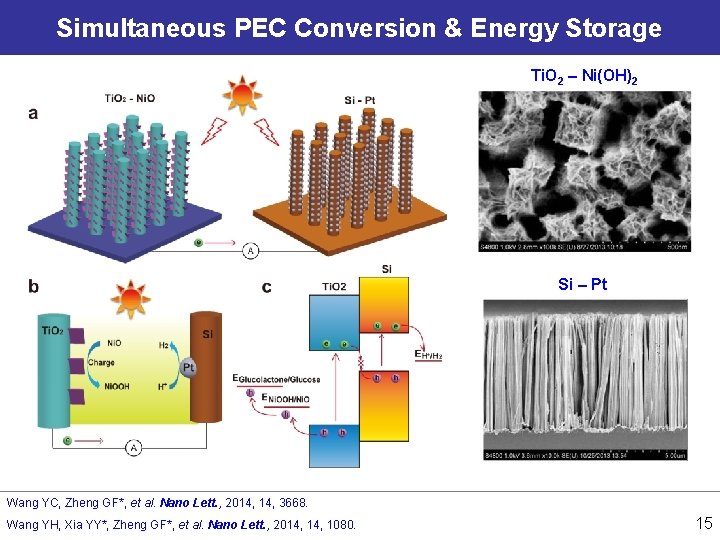
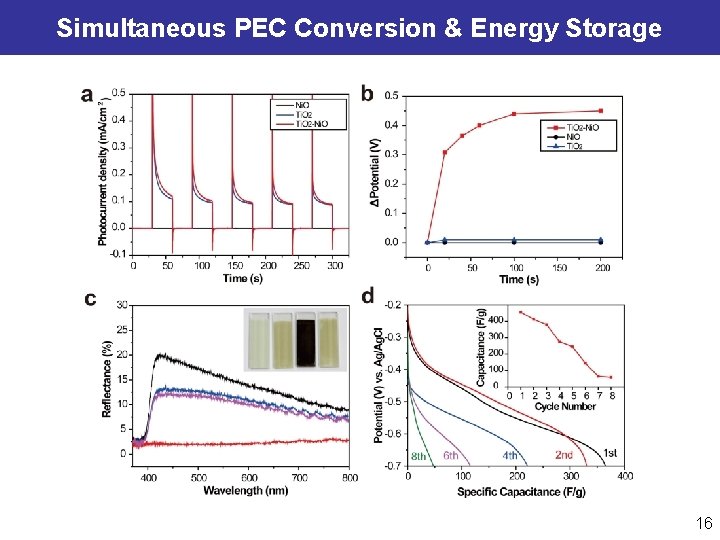
Simultaneous PEC Conversion & Energy Storage Ti. O 2 – Ni(OH)2 Si – Pt Wang YC, Zheng GF*, et al. Nano Lett. , 2014, 3668. Wang YH, Xia YY*, Zheng GF*, et al. Nano Lett. , 2014, 1080. 15

Simultaneous PEC Conversion & Energy Storage 16

Research Design ηtotal = ηabsorption × ηseparation × ηtransfer Bandgap Tuning Charge Carrier Density Charge Transfer 17

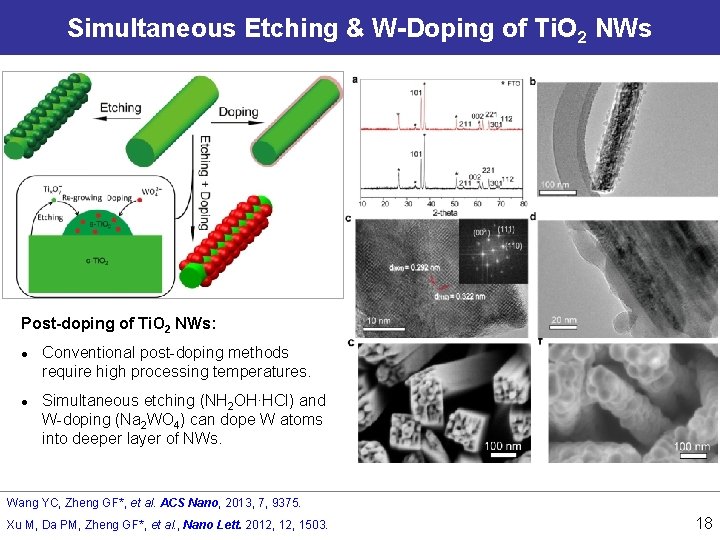
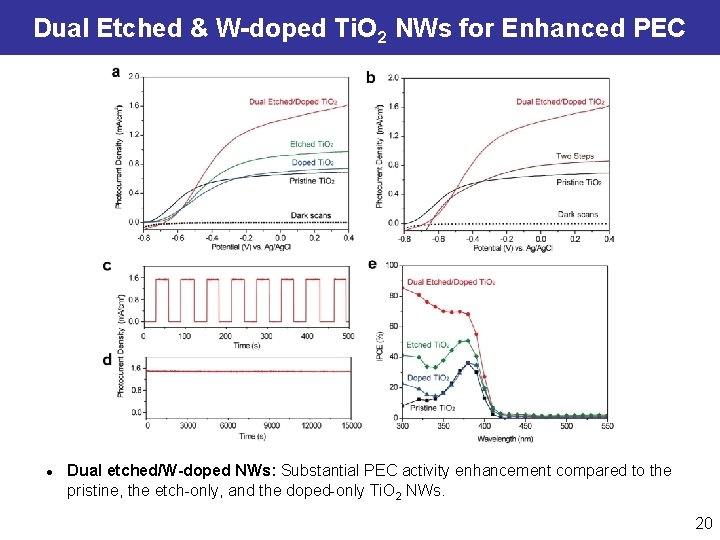
Simultaneous Etching & W-Doping of Ti. O 2 NWs Post-doping of Ti. O 2 NWs: l l Conventional post-doping methods require high processing temperatures. Simultaneous etching (NH 2 OH∙HCl) and W-doping (Na 2 WO 4) can dope W atoms into deeper layer of NWs. Wang YC, Zheng GF*, et al. ACS Nano, 2013, 7, 9375. Xu M, Da PM, Zheng GF*, et al. , Nano Lett. 2012, 1503. 18

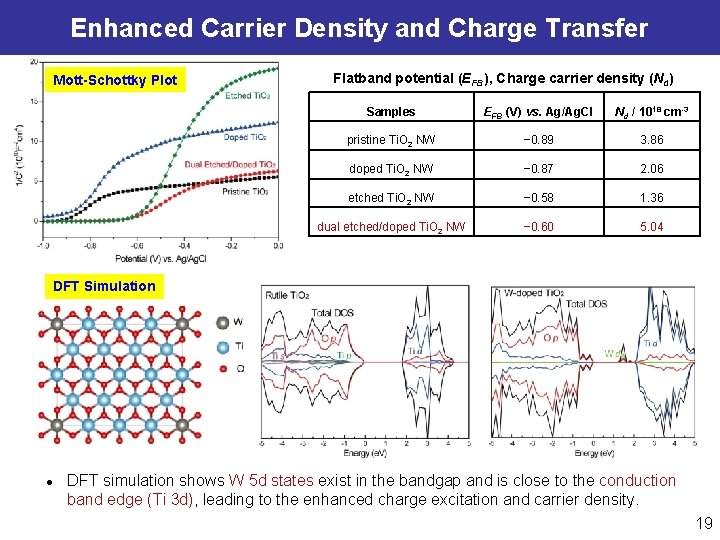
Enhanced Carrier Density and Charge Transfer Mott-Schottky Plot Flatband potential (EFB), Charge carrier density (Nd) Samples EFB (V) vs. Ag/Ag. Cl Nd / 1018 cm-3 pristine Ti. O 2 NW − 0. 89 3. 86 doped Ti. O 2 NW − 0. 87 2. 06 etched Ti. O 2 NW − 0. 58 1. 36 dual etched/doped Ti. O 2 NW − 0. 60 5. 04 DFT Simulation l DFT simulation shows W 5 d states exist in the bandgap and is close to the conduction band edge (Ti 3 d), leading to the enhanced charge excitation and carrier density. 19

Dual Etched & W-doped Ti. O 2 NWs for Enhanced PEC l Dual etched/W-doped NWs: Substantial PEC activity enhancement compared to the pristine, the etch-only, and the doped-only Ti. O 2 NWs. 20

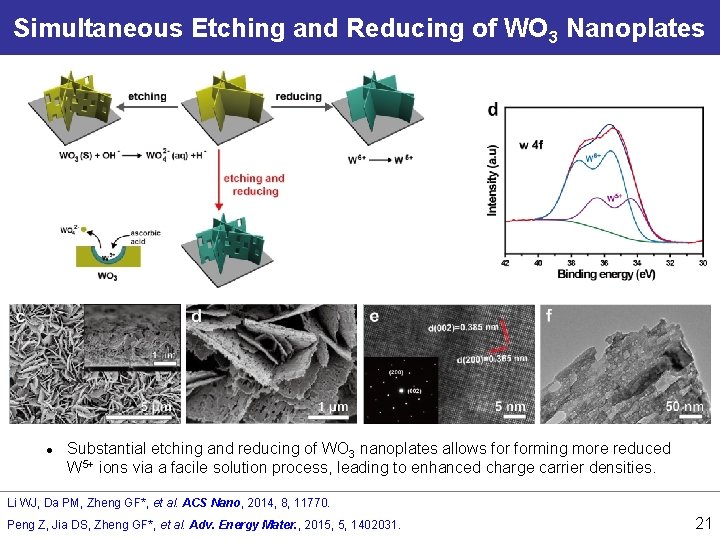
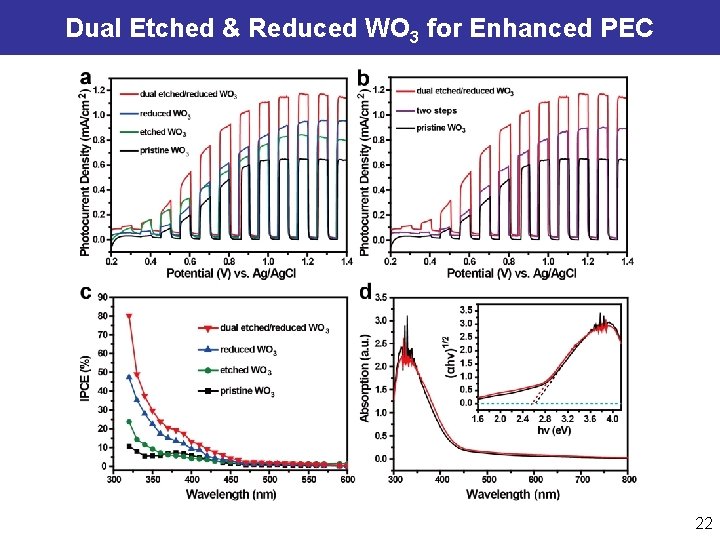
Simultaneous Etching and Reducing of WO 3 Nanoplates l Substantial etching and reducing of WO 3 nanoplates allows forming more reduced W 5+ ions via a facile solution process, leading to enhanced charge carrier densities. Li WJ, Da PM, Zheng GF*, et al. ACS Nano, 2014, 8, 11770. Peng Z, Jia DS, Zheng GF*, et al. Adv. Energy Mater. , 2015, 5, 1402031. 21

Dual Etched & Reduced WO 3 for Enhanced PEC 22

Research Design ηtotal = ηabsorption × ηseparation × ηtransfer Bandgap Tuning Charge Carrier Density Charge Transfer 23

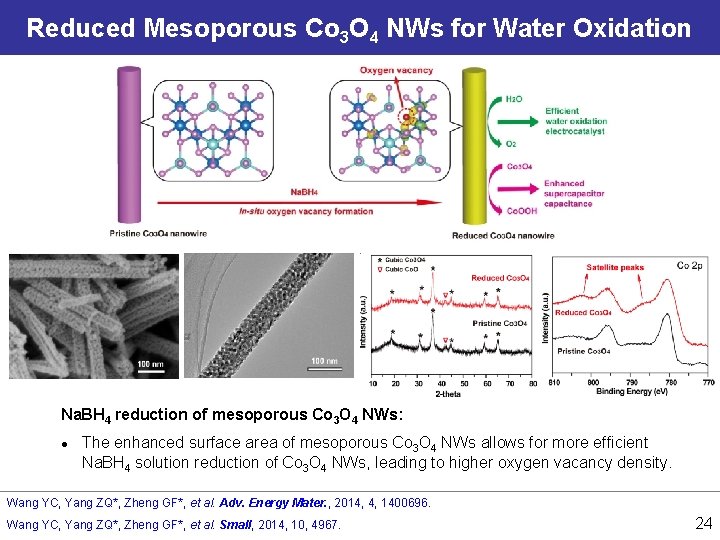
Reduced Mesoporous Co 3 O 4 NWs for Water Oxidation Na. BH 4 reduction of mesoporous Co 3 O 4 NWs: l The enhanced surface area of mesoporous Co 3 O 4 NWs allows for more efficient Na. BH 4 solution reduction of Co 3 O 4 NWs, leading to higher oxygen vacancy density. Wang YC, Yang ZQ*, Zheng GF*, et al. Adv. Energy Mater. , 2014, 4, 1400696. Wang YC, Yang ZQ*, Zheng GF*, et al. Small, 2014, 10, 4967. 24

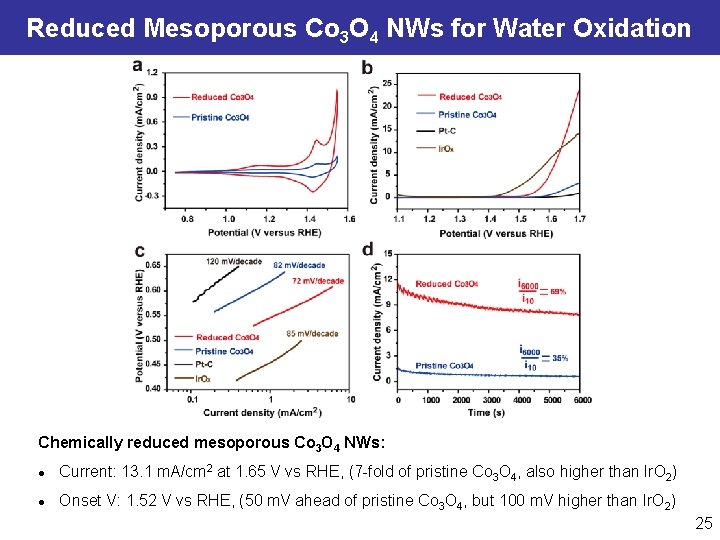
Reduced Mesoporous Co 3 O 4 NWs for Water Oxidation Chemically reduced mesoporous Co 3 O 4 NWs: l Current: 13. 1 m. A/cm 2 at 1. 65 V vs RHE, (7 -fold of pristine Co 3 O 4, also higher than Ir. O 2) l Onset V: 1. 52 V vs RHE, (50 m. V ahead of pristine Co 3 O 4, but 100 m. V higher than Ir. O 2) 25

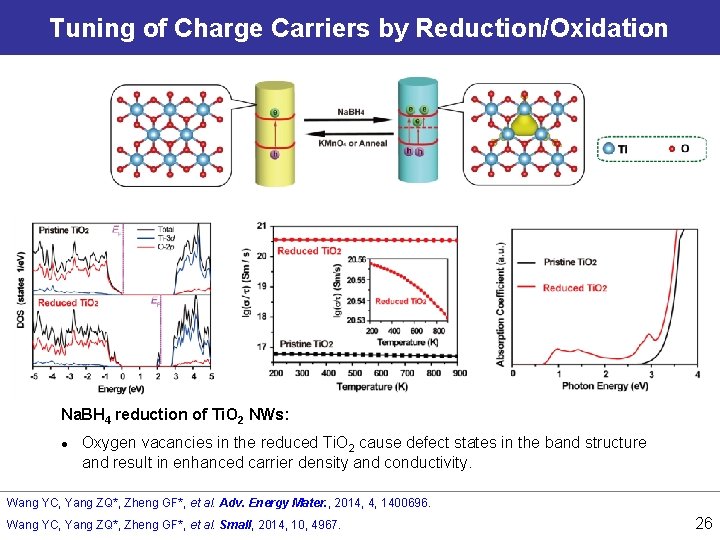
Tuning of Charge Carriers by Reduction/Oxidation Na. BH 4 reduction of Ti. O 2 NWs: l Oxygen vacancies in the reduced Ti. O 2 cause defect states in the band structure and result in enhanced carrier density and conductivity. Wang YC, Yang ZQ*, Zheng GF*, et al. Adv. Energy Mater. , 2014, 4, 1400696. Wang YC, Yang ZQ*, Zheng GF*, et al. Small, 2014, 10, 4967. 26

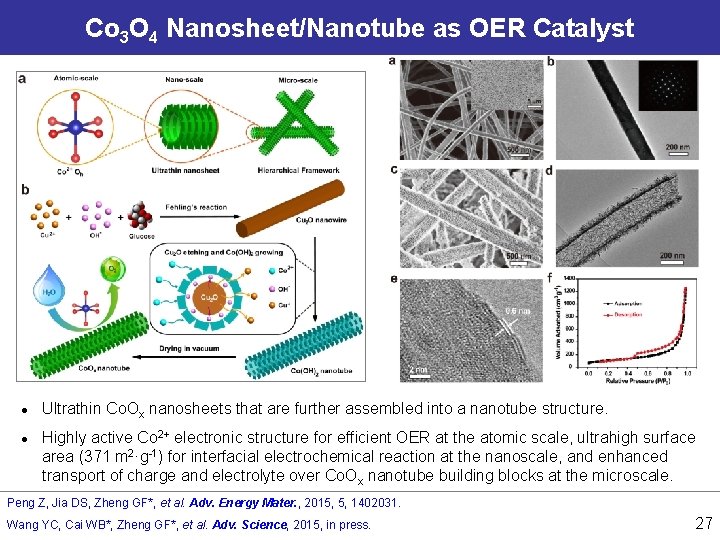
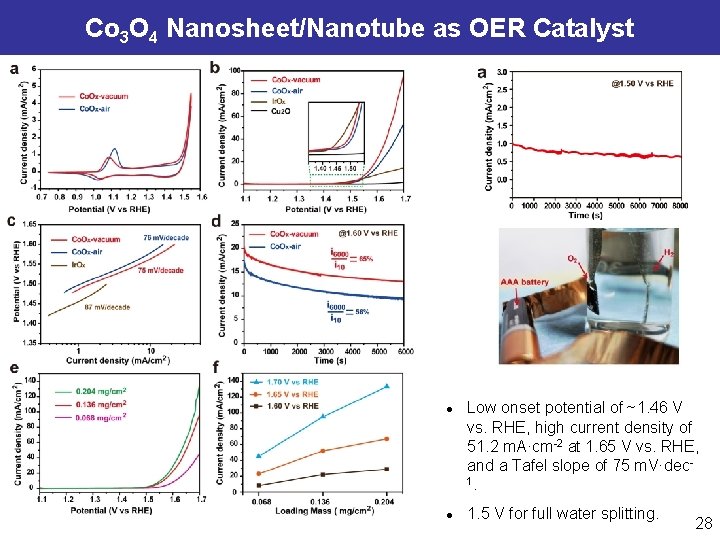
Co 3 O 4 Nanosheet/Nanotube as OER Catalyst l l Ultrathin Co. Ox nanosheets that are further assembled into a nanotube structure. Highly active Co 2+ electronic structure for efficient OER at the atomic scale, ultrahigh surface area (371 m 2·g-1) for interfacial electrochemical reaction at the nanoscale, and enhanced transport of charge and electrolyte over Co. Ox nanotube building blocks at the microscale. Peng Z, Jia DS, Zheng GF*, et al. Adv. Energy Mater. , 2015, 5, 1402031. Wang YC, Cai WB*, Zheng GF*, et al. Adv. Science, 2015, in press. 27

Co 3 O 4 Nanosheet/Nanotube as OER Catalyst l l Low onset potential of ~1. 46 V vs. RHE, high current density of 51. 2 m. A·cm-2 at 1. 65 V vs. RHE, and a Tafel slope of 75 m. V·dec 1. 1. 5 V for full water splitting. 28

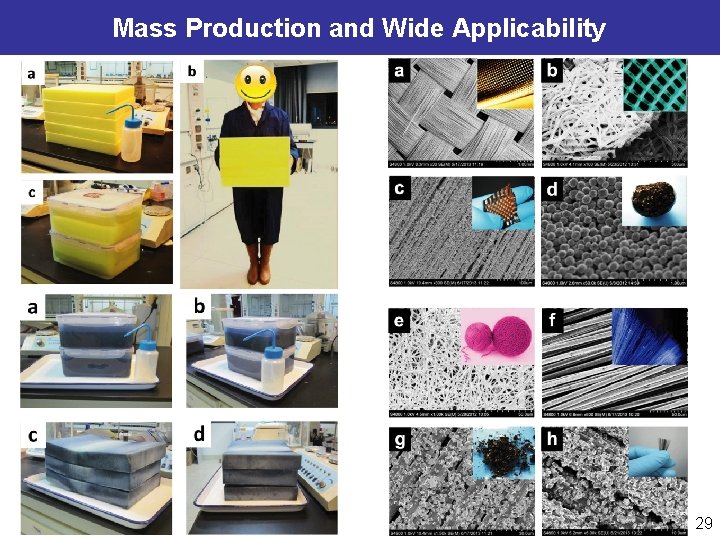
Mass Production and Wide Applicability 29

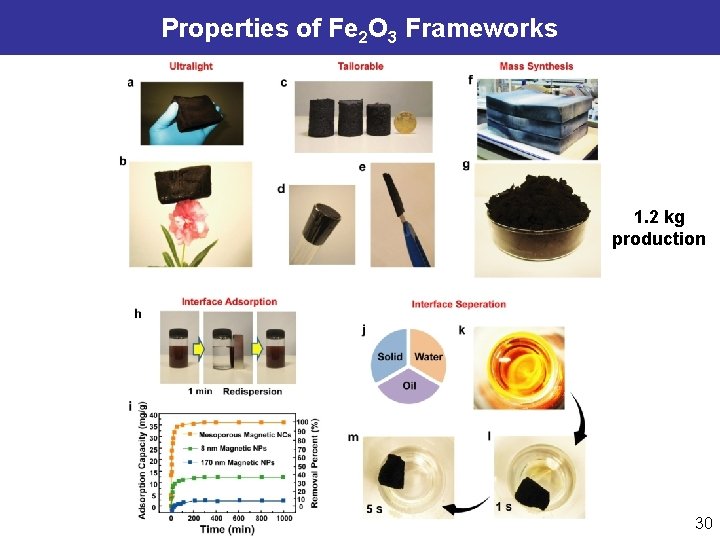
Properties of Fe 2 O 3 Frameworks 1. 2 kg production 30

3. 8 m 31

Research Goal Research in synthesis of new nanomaterials/structures and developing novel physical measurement methods can open a variety of opportunities for catalysis, photoconversion, energy storage and biointerface. Review: Wang YL, Zheng GF*, et al. Adv. Mater. 2013, 25, 5177. Review: Tang J, Li J, Zheng GF*, et al. Chem. Eur. J. 2015, in press. 32

Thank You ! Shanghai – Seen from a Clear Sky 33