SPECTROSCOPIC AND KINETIC MEASUREMENTS OF ORGANIC PEROXY RADICALS




- Slides: 19

SPECTROSCOPIC AND KINETIC MEASUREMENTS OF ORGANIC PEROXY RADICALS BY DUAL-WAVELENGTH CAVITY RING DOWN SPECTROSCOPY DMITRY G. MELNIK AND TERRY A. MILLER The Ohio State University, Dept. of Chemistry, Laser Spectroscopy Facility, 120 W. 18 th Avenue, Columbus, Ohio 43210

Motivation 1. Peroxy radicals play central role in combustion chemisrty. 2. For quantitative monitoring, understanding and managing combustion and atmospheric processes one needs information on physical (absorption cross-section) and chemical properties (relevant reaction rate constants) 3. Present day data suffer from large error bars, and possibly substantial systematic errors 4. Cross-species reaction rates are particularly important for understanding chemical processes in complex environment.

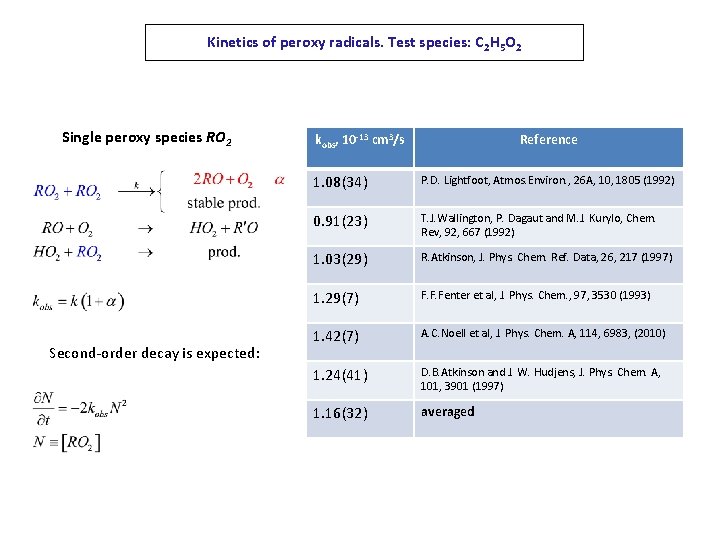
Kinetics of peroxy radicals. Test species: C 2 H 5 O 2 Single peroxy species RO 2 Second-order decay is expected: kobs, 10 -13 cm 3/s Reference 1. 08(34) P. D. Lightfoot, Atmos. Environ. , 26 A, 10, 1805 (1992) 0. 91(23) T. J. Wallington, P. Dagaut and M. J. Kurylo, Chem. Rev, 92, 667 (1992) 1. 03(29) R. Atkinson, J. Phys. Chem. Ref. Data, 26, 217 (1997) 1. 29(7) F. F. Fenter et al, J. Phys. Chem. , 97, 3530 (1993) 1. 42(7) A. C. Noell et al, J. Phys. Chem. A, 114, 6983, (2010) 1. 24(41) D. B. Atkinson and J. W. Hudjens, J. Phys. Chem. A, 101, 3901 (1997) 1. 16(32) averaged

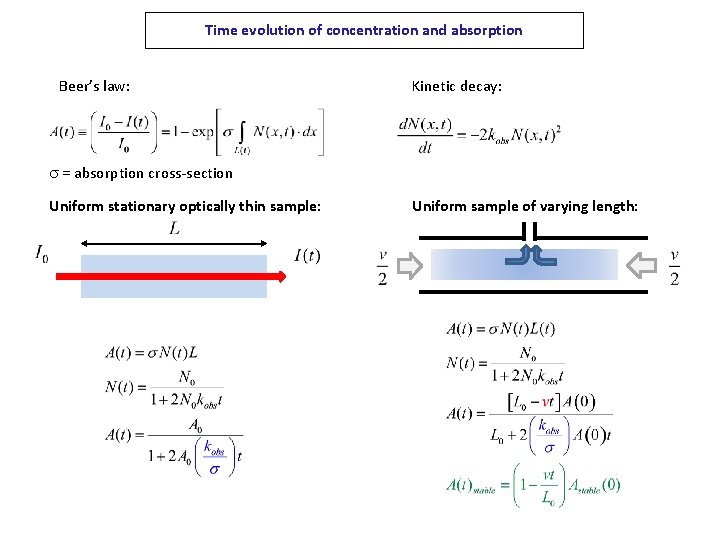
Time evolution of concentration and absorption Beer’s law: Kinetic decay: s = absorption cross-section Uniform stationary optically thin sample: Uniform sample of varying length:

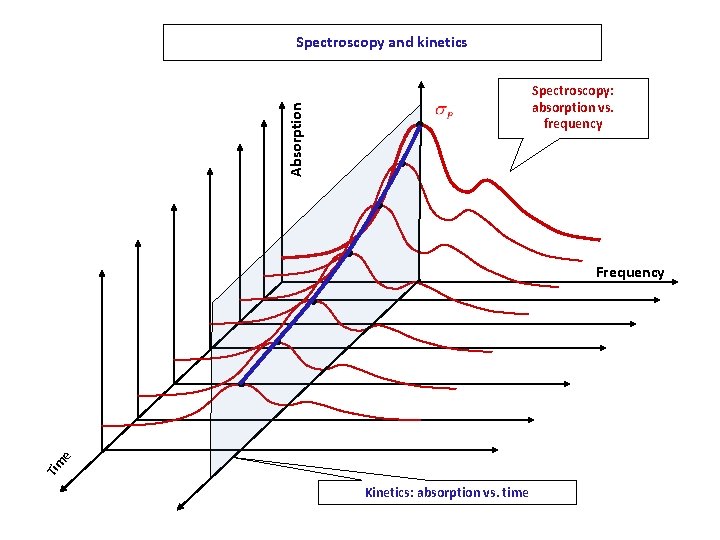
Spectroscopy and kinetics Absorption Spectroscopy: absorption vs. frequency Tim e Frequency Kinetics: absorption vs. time

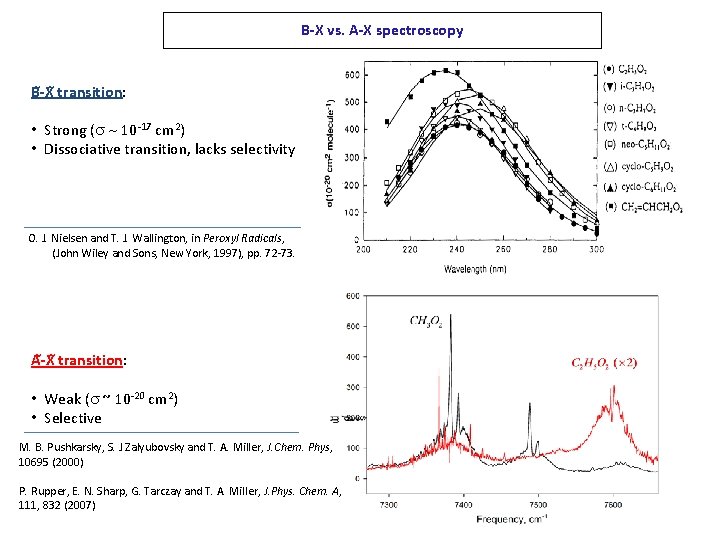
B-X vs. A-X spectroscopy B -X transition: • Strong (s ~ 10 -17 cm 2) • Dissociative transition, lacks selectivity O. J. Nielsen and T. J. Wallington, in Peroxyl Radicals, (John Wiley and Sons, New York, 1997), pp. 72 -73. A -X transition: • Weak (s ~ 10 -20 cm 2) • Selective M. B. Pushkarsky, S. J Zalyubovsky and T. A. Miller, J. Chem. Phys, 10695 (2000) P. Rupper, E. N. Sharp, G. Tarczay and T. A. Miller, J. Phys. Chem. A, 111, 832 (2007)

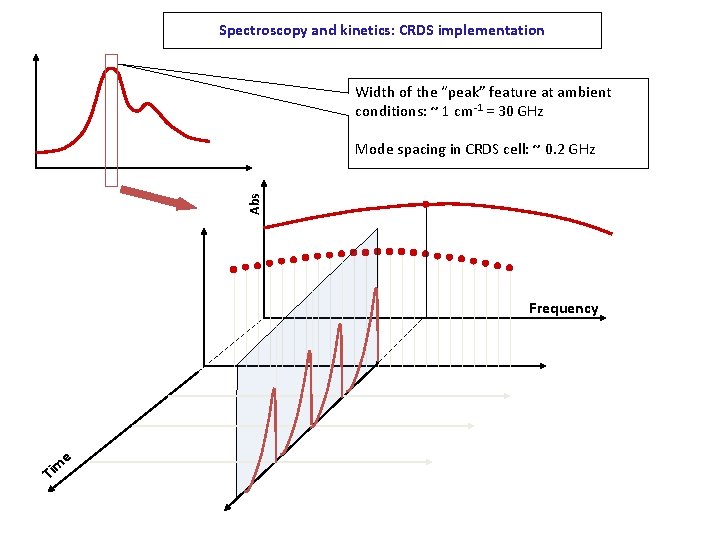
Spectroscopy and kinetics: CRDS implementation Width of the “peak” feature at ambient conditions: ~ 1 cm-1 = 30 GHz Abs Mode spacing in CRDS cell: ~ 0. 2 GHz Frequency e Tim

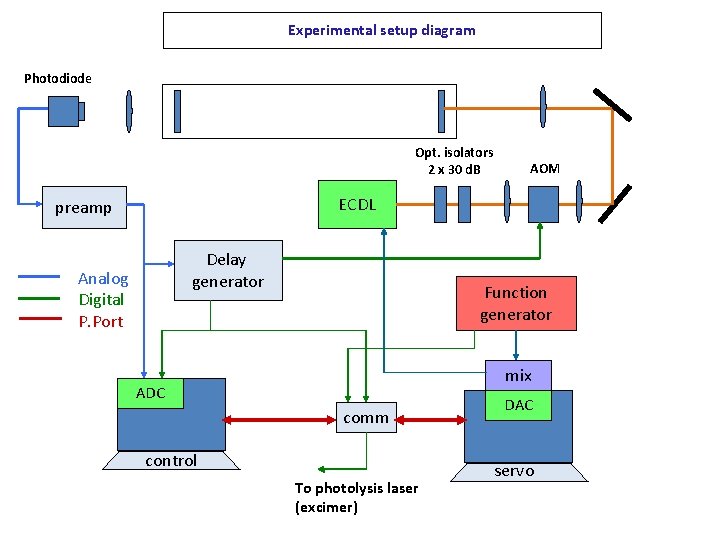
Experimental setup diagram Photodiode Opt. isolators 2 x 30 d. B AOM ECDL preamp Delay generator Analog Digital P. Port Function generator mix ADC comm control To photolysis laser (excimer) DAC servo

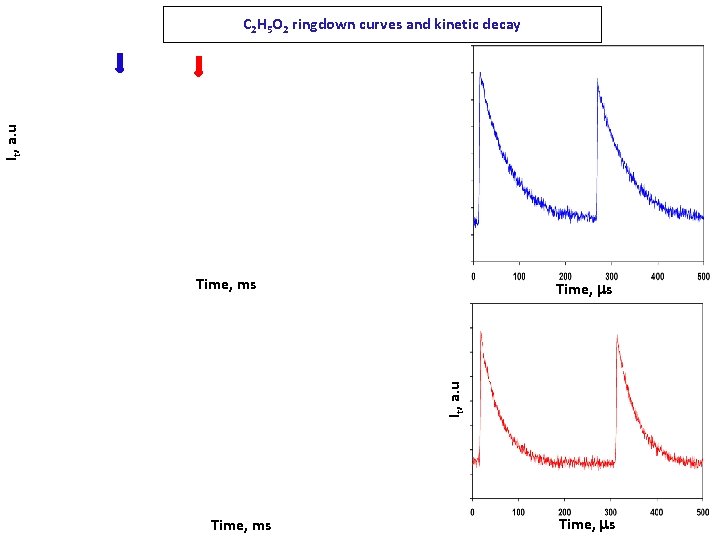
It, a. u C 2 H 5 O 2 ringdown curves and kinetic decay Time, ms It, a. u Time, ms

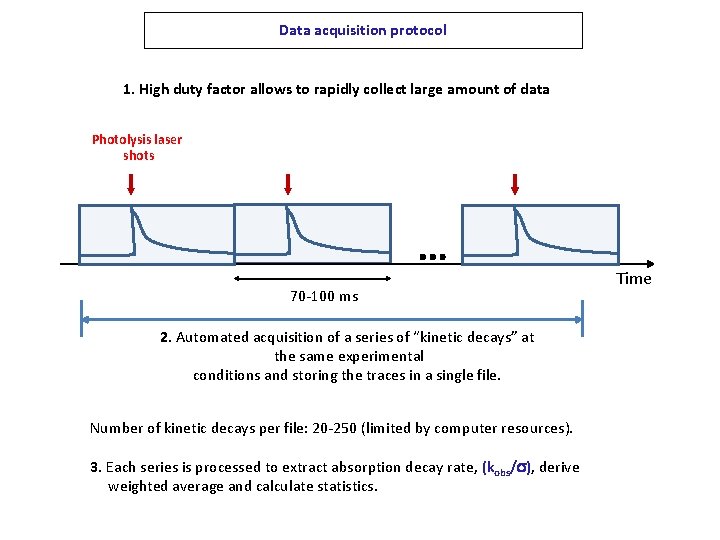
Data acquisition protocol 1. High duty factor allows to rapidly collect large amount of data Photolysis laser shots … 70 -100 ms 2. Automated acquisition of a series of “kinetic decays” at the same experimental conditions and storing the traces in a single file. Number of kinetic decays per file: 20 -250 (limited by computer resources). 3. Each series is processed to extract absorption decay rate, (kobs/s), derive weighted average and calculate statistics. Time

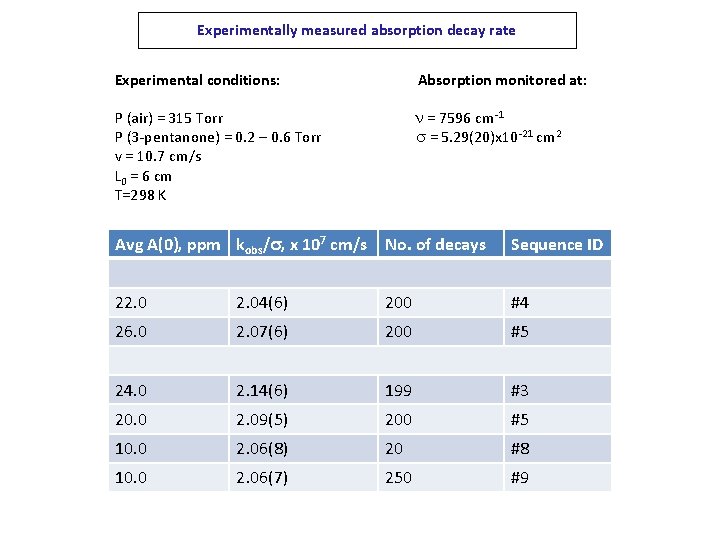
Experimentally measured absorption decay rate Experimental conditions: Absorption monitored at: P (air) = 315 Torr P (3 -pentanone) = 0. 2 – 0. 6 Torr v = 10. 7 cm/s L 0 = 6 cm T=298 K n = 7596 cm-1 s = 5. 29(20)x 10 -21 cm 2 Avg A(0), ppm kobs/s, x 107 cm/s No. of decays Sequence ID 22. 04(6) 200 #4 26. 0 2. 07(6) 200 #5 24. 0 2. 14(6) 199 #3 20. 0 2. 09(5) 200 #5 10. 0 2. 06(8) 20 #8 10. 0 2. 06(7) 250 #9

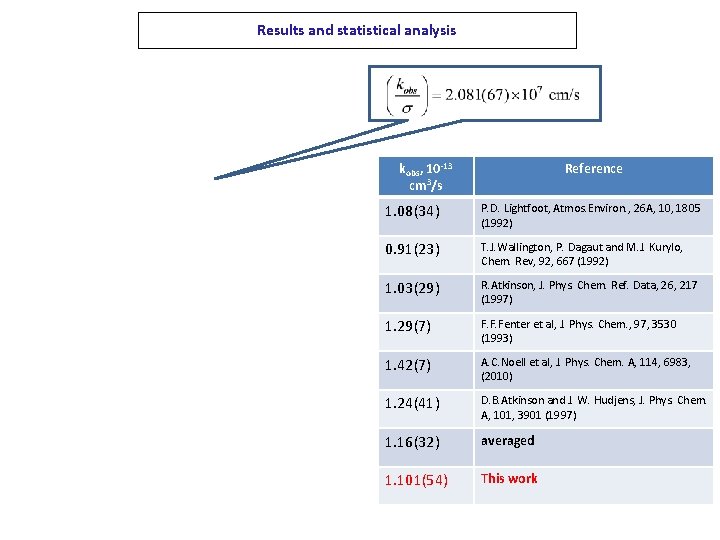
Results and statistical analysis kobs, 10 -13 cm 3/s Reference 1. 08(34) P. D. Lightfoot, Atmos. Environ. , 26 A, 10, 1805 (1992) 0. 91(23) T. J. Wallington, P. Dagaut and M. J. Kurylo, Chem. Rev, 92, 667 (1992) 1. 03(29) R. Atkinson, J. Phys. Chem. Ref. Data, 26, 217 (1997) 1. 29(7) F. F. Fenter et al, J. Phys. Chem. , 97, 3530 (1993) 1. 42(7) A. C. Noell et al, J. Phys. Chem. A, 114, 6983, (2010) 1. 24(41) D. B. Atkinson and J. W. Hudjens, J. Phys. Chem. A, 101, 3901 (1997) 1. 16(32) averaged 1. 101(54) This work

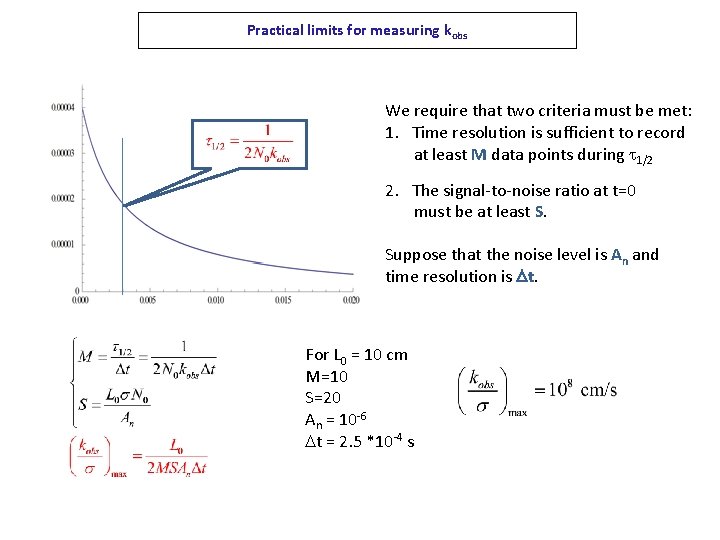
Practical limits for measuring kobs We require that two criteria must be met: 1. Time resolution is sufficient to record at least M data points during t 1/2 2. The signal-to-noise ratio at t=0 must be at least S. Suppose that the noise level is An and time resolution is Dt. For L 0 = 10 cm M=10 S=20 An = 10 -6 Dt = 2. 5 *10 -4 s

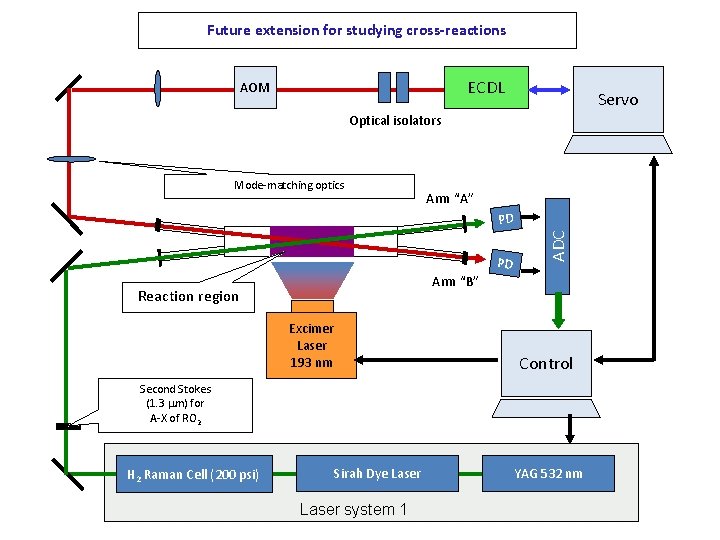
Future extension for studying cross-reactions ECDL AOM Servo Optical isolators Mode-matching optics Arm “A” PD ADC PD Arm “B” Reaction region Excimer Laser 193 nm Control Second Stokes (1. 3 mm) for A-X of RO 2 H 2 Raman Cell (200 psi) Sirah Dye Laser system 1 YAG 532 nm

Summary 1. We have built a CW-CRDS based apparatus capable of a. following the kinetic decay of the same set of radicals with time resolution ~ 250 ms. b. rapid data acquisition due to high duty factor. c. capable, at present, measuring absorption decay rates, (kobs/s), up to about 108 cm/s which is suitable for studying kinetics of peroxy radiucals. 2. We have measured the absorption decay rate and the rate of removal of ethyl peroxy radicals due to self-reaction, 3. The presented experimental method can be straightforwardly extended to study of the rate constants of the reactions between different radicals.

Acknowledgements • Colleagues: Dr. Mourad Roudjane Dr. Takashige Fujiwara Dr. Dianping Sun Terrance Codd, Neal Kline Rabi Chhantyal Pun

Supplementary slides

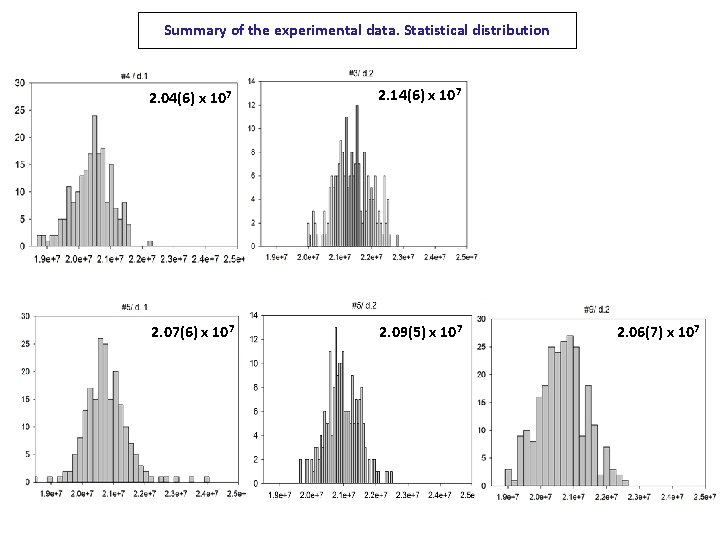
Summary of the experimental data. Statistical distribution 2. 04(6) x 107 2. 14(6) x 107 2. 07(6) x 107 2. 09(5) x 107 2. 06(7) x 107

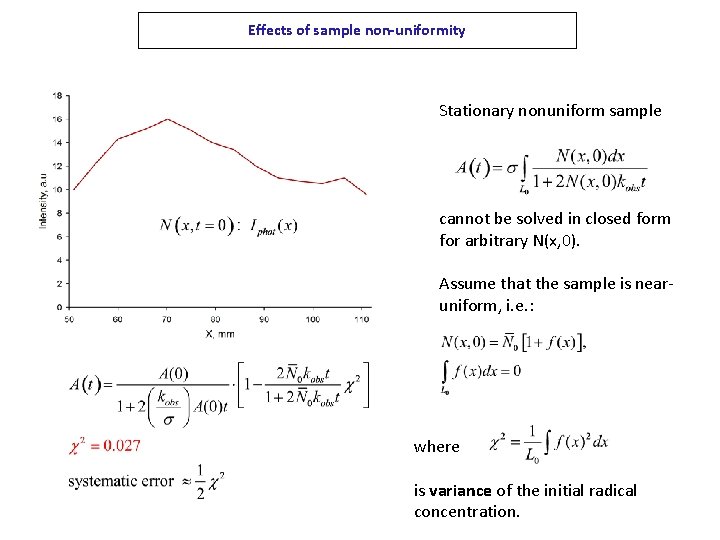
Effects of sample non-uniformity Stationary nonuniform sample cannot be solved in closed form for arbitrary N(x, 0). Assume that the sample is nearuniform, i. e. : where is variance of the initial radical concentration.
 Unlike radicals examples
Unlike radicals examples How to convert mixed radicals to entire radicals
How to convert mixed radicals to entire radicals Spectroscopic notation
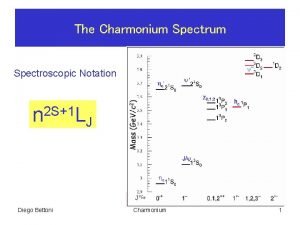
Spectroscopic notation Spectroscopic data in chemistry
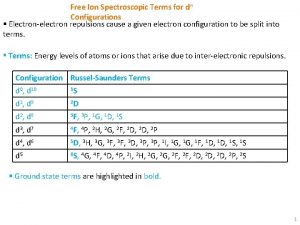
Spectroscopic data in chemistry Free ion term
Free ion term In chromatography
In chromatography Adding subtracting and multiplying radicals
Adding subtracting and multiplying radicals Square root of 72 in radical form
Square root of 72 in radical form Complex numbers and rational exponents
Complex numbers and rational exponents Solving equations with radicals and rational exponents
Solving equations with radicals and rational exponents Lesson 0-9 square roots and simplifying radicals answers
Lesson 0-9 square roots and simplifying radicals answers Radicals and pythagorean theorem
Radicals and pythagorean theorem Quiz 6-1 radicals and rational exponents answers
Quiz 6-1 radicals and rational exponents answers Measurements equivalents and adjustments
Measurements equivalents and adjustments Chapter 2 measurements and calculations
Chapter 2 measurements and calculations Force and torque measurements
Force and torque measurements Ee8403 measurements and instrumentation
Ee8403 measurements and instrumentation Vital signs and anthropometric measurements
Vital signs and anthropometric measurements Vital signs and measurements chapter 37
Vital signs and measurements chapter 37 Finding segment lengths in circles
Finding segment lengths in circles