A New Spectroscopic Window on Hydroxyl Radicals and



- Slides: 27

A New Spectroscopic Window on Hydroxyl Radicals and their Association Reactions of Significance in the Atmosphere Marsha I. Lester University of Pennsylvania Ø Association reactions of OH with atmospheric partners Ø New photoionization scheme for OH detection Molecular Spectroscopy Symposium June 18, 2012 National Science Foundation Department of Energy

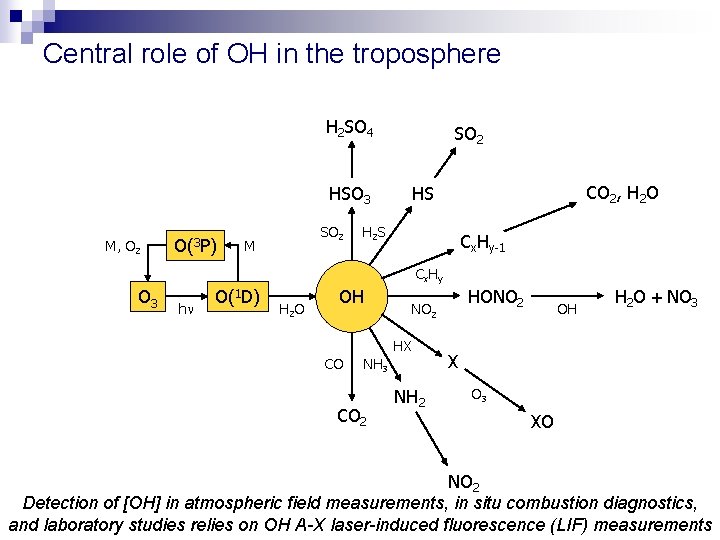
Central role of OH in the troposphere H 2 SO 4 HSO 3 M, O 2 O(3 P) SO 2 M SO 2 CO 2, H 2 O HS H 2 S Cx. Hy-1 C x Hy O 3 h O(1 D) H 2 O OH HX CO HONO 2 NH 3 CO 2 NH 2 OH H 2 O + NO 3 XO NO 2 Detection of [OH] in atmospheric field measurements, in situ combustion diagnostics, and laboratory studies relies on OH A-X laser-induced fluorescence (LIF) measurements

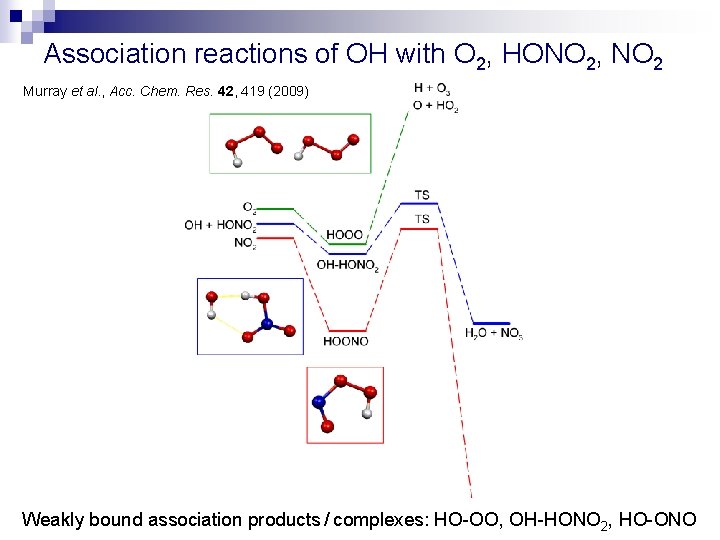
Association reactions of OH with O 2, HONO 2, NO 2 Murray et al. , Acc. Chem. Res. 42, 419 (2009) Weakly bound association products / complexes: HO-OO, OH-HONO 2, HO-ONO

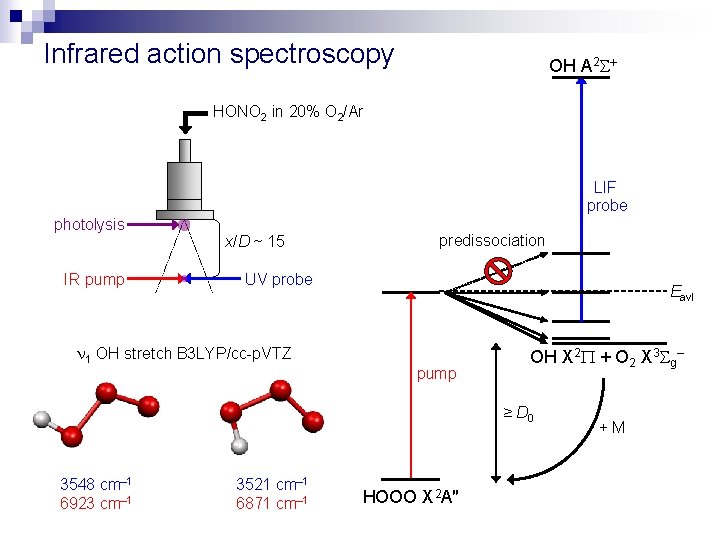
Infrared action spectroscopy OH A 2 HONO 2 in 20% O 2/Ar LIF probe photolysis IR pump x/D ~ 15 predissociation UV probe 1 OH stretch B 3 LYP/cc-p. VTZ Eavl pump OH X 2 + O 2 X 3 g– ≥ D 0 3548 cm– 1 6923 cm– 1 3521 cm– 1 6871 cm– 1 HOOO X 2 A″ +M

IR action spectra of HOOO Probe OH A–X (1, 0) P 1(4) transition Probe OH A–X (1, 1) P 1(4) transition 1 2 1 Structured bands simulated with FTMW rotational constants for trans-HOOO r. O-O = 1. 688 Å Suma et al. , Science 308, 1885 (2005) Unstructured features attributed to cis-HOOO Mc. Carthy et al. , J. Chem. Phys. 136, 034303 (2012) Total HOOO simulation Derro et al. , J. Phys. Chem. A 111, 11592 (2007)

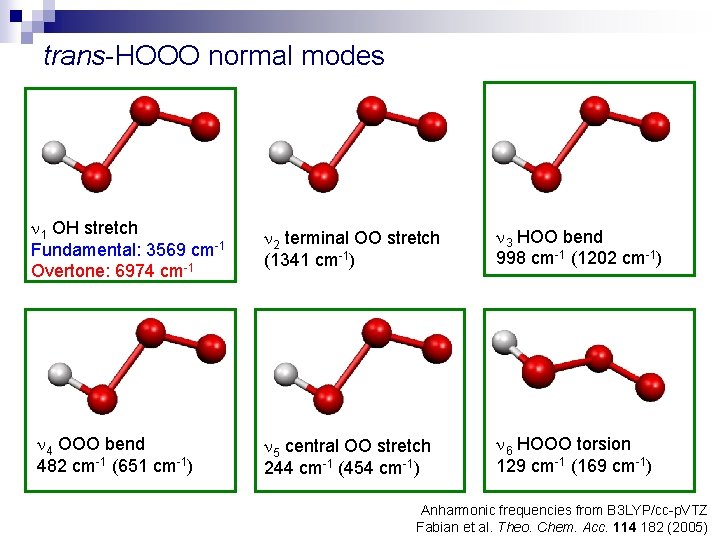
trans-HOOO normal modes 1 OH stretch Fundamental: 3569 cm-1 Overtone: 6974 cm-1 4 OOO bend 482 cm-1 (651 cm-1) 2 terminal OO stretch (1341 cm-1) 3 HOO bend 998 cm-1 (1202 cm-1) 5 central OO stretch 244 cm-1 (454 cm-1) 6 HOOO torsion 129 cm-1 (169 cm-1) Anharmonic frequencies from B 3 LYP/cc-p. VTZ Fabian et al. Theo. Chem. Acc. 114 182 (2005)

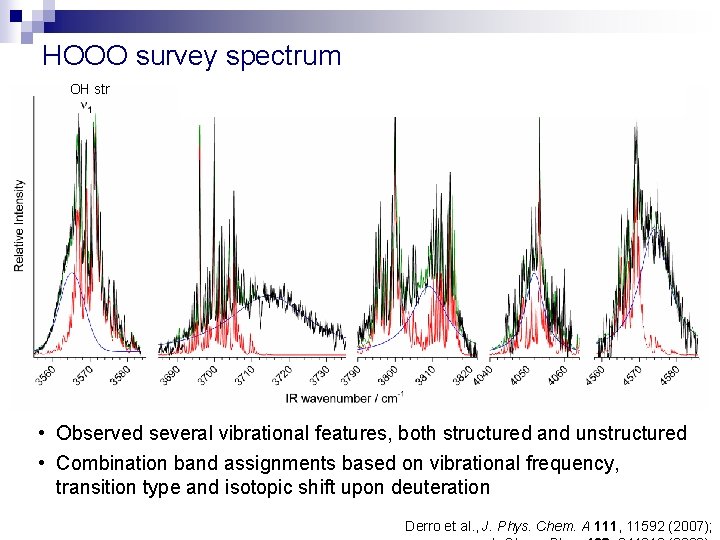
HOOO survey spectrum OH str torsion OOO bend OO str HOO bend • Observed several vibrational features, both structured and unstructured • Combination band assignments based on vibrational frequency, transition type and isotopic shift upon deuteration Derro et al. , J. Phys. Chem. A 111, 11592 (2007);

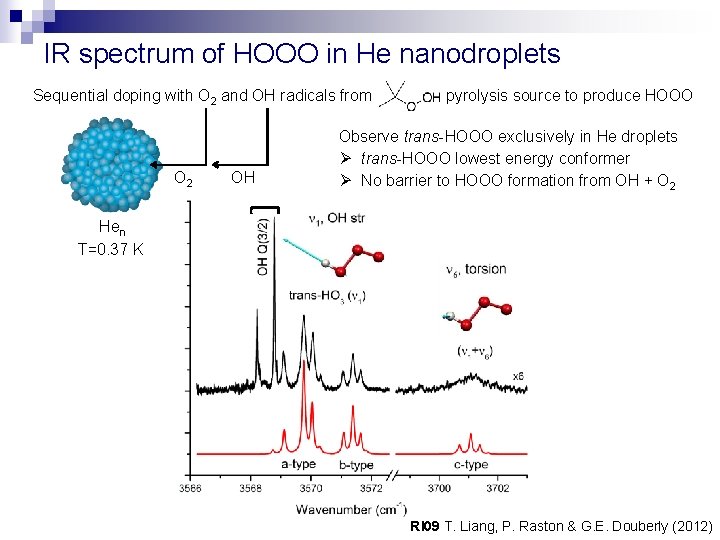
IR spectrum of HOOO in He nanodroplets Sequential doping with O 2 and OH radicals from O 2 OH pyrolysis source to produce HOOO Observe trans-HOOO exclusively in He droplets Ø trans-HOOO lowest energy conformer Ø No barrier to HOOO formation from OH + O 2 Hen T=0. 37 K RI 09 T. Liang, P. Raston & G. E. Douberly (2012)

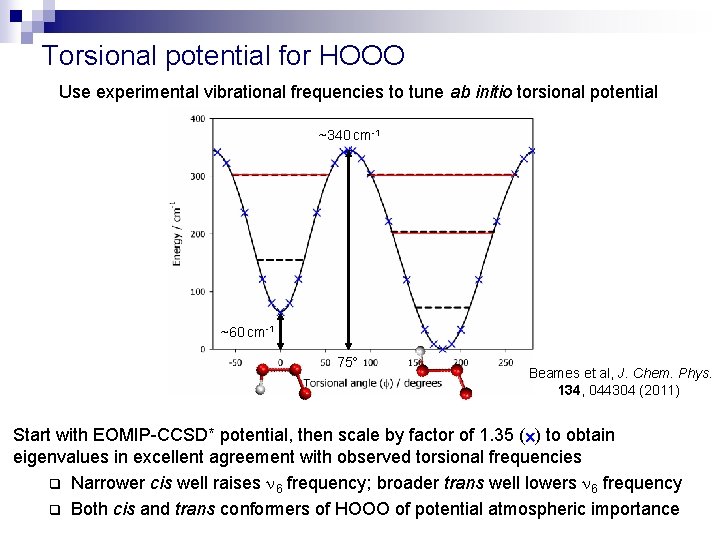
Torsional potential for HOOO Use experimental vibrational frequencies to tune ab initio torsional potential ~340 cm-1 ~60 cm-1 75 Beames et al, J. Chem. Phys. 134, 044304 (2011) Start with EOMIP-CCSD* potential, then scale by factor of 1. 35 ( ) to obtain eigenvalues in excellent agreement with observed torsional frequencies q Narrower cis well raises 6 frequency; broader trans well lowers 6 frequency q Both cis and trans conformers of HOOO of potential atmospheric importance

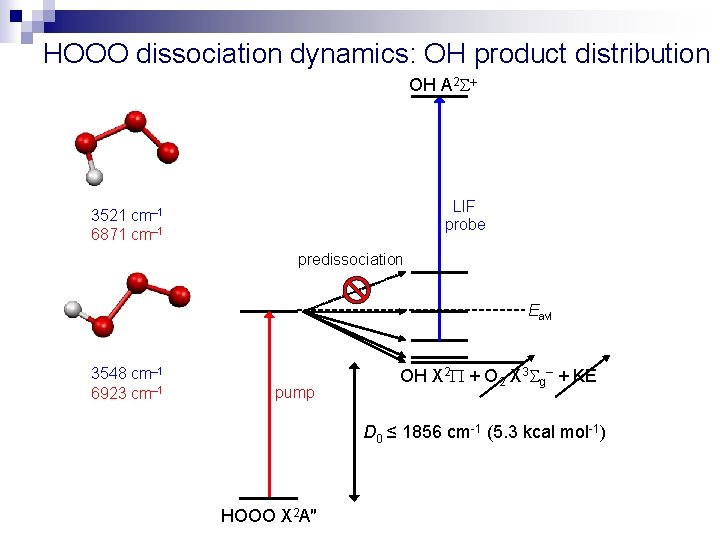
HOOO dissociation dynamics: OH product distribution OH A 2 LIF probe 3521 cm– 1 6871 cm– 1 predissociation Eavl 3548 cm– 1 6923 cm– 1 pump OH X 2 + O 2 X 3 g– + KE D 0 ≤ 1856 cm-1 (5. 3 kcal mol-1) HOOO X 2 A″

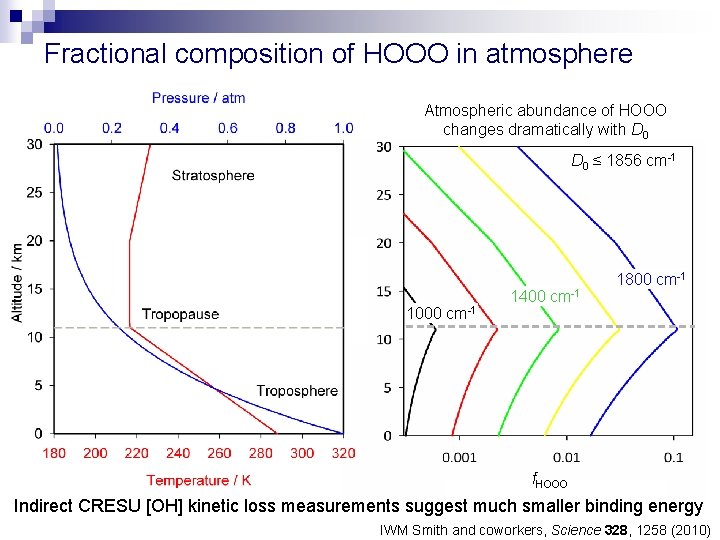
Fractional composition of HOOO in atmosphere Atmospheric abundance of HOOO changes dramatically with D 0 ≤ 1856 cm-1 1000 cm-1 1400 cm-1 1800 cm-1 f. HOOO Indirect CRESU [OH] kinetic loss measurements suggest much smaller binding energy IWM Smith and coworkers, Science 328, 1258 (2010)

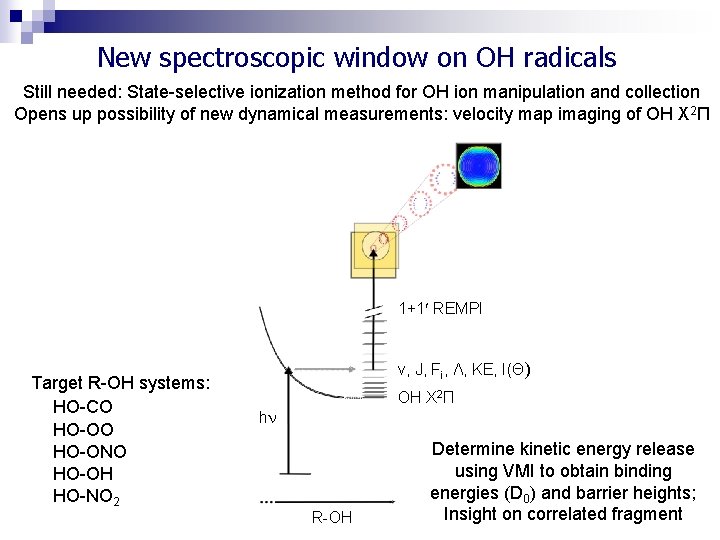
New spectroscopic window on OH radicals Still needed: State-selective ionization method for OH ion manipulation and collection Opens up possibility of new dynamical measurements: velocity map imaging of OH X 2Π 1+1 REMPI Target R-OH systems: HO-CO HO-ONO HO-OH HO-NO 2 v, J, Fi, Λ, KE, I(Θ) OH X 2Π h R-OH Determine kinetic energy release using VMI to obtain binding energies (D 0) and barrier heights; Insight on correlated fragment

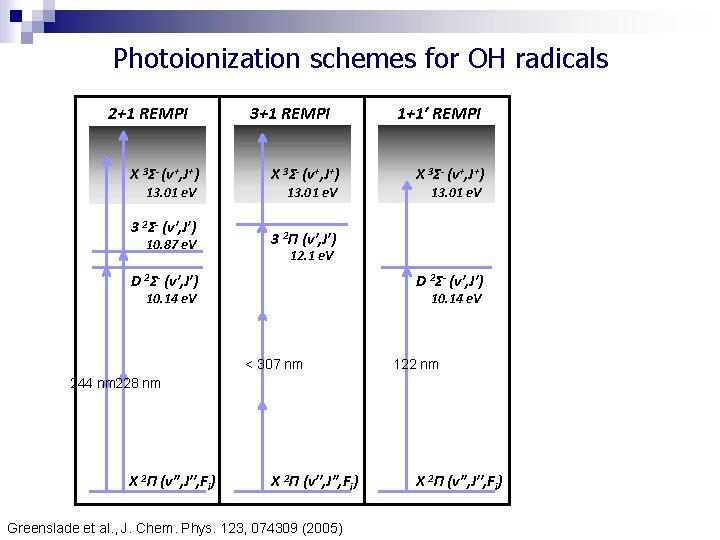
Photoionization schemes for OH radicals 2+1 REMPI X 3Σ- (v+, J+) 13. 01 e. V 3 2Σ- (v′, J′) 10. 87 e. V 3+1 REMPI X 3Σ- (v+, J+) 13. 01 e. V 1+1′ REMPI X 3Σ- (v+, J+) 13. 01 e. V 3 2Π (v′, J′) 12. 1 e. V D 2Σ- (v′, J′) 10. 14 e. V < 307 nm 122 nm 244 nm 228 nm X 2Π (v′′, J′′, Fi) Greenslade et al. , J. Chem. Phys. 123, 074309 (2005) X 2Π (v′′, J′′, Fi)

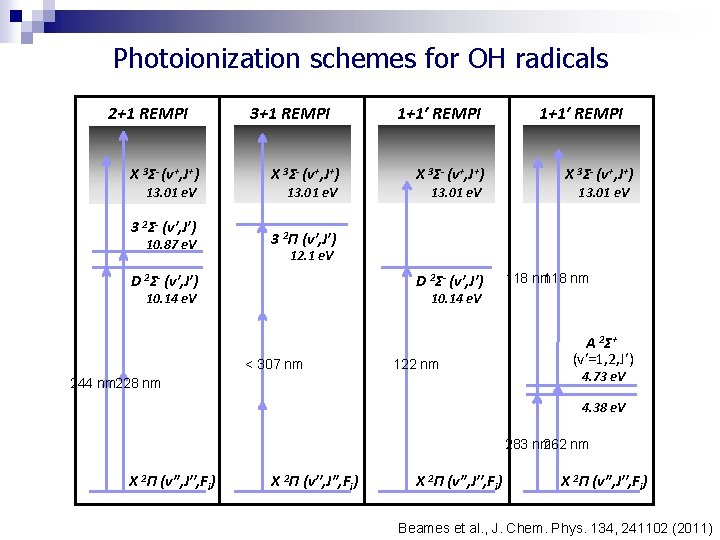
Photoionization schemes for OH radicals 2+1 REMPI X 3Σ- (v+, J+) 13. 01 e. V 3 2Σ- (v′, J′) 10. 87 e. V 3+1 REMPI X 3Σ- (v+, J+) 13. 01 e. V 1+1′ REMPI X 3Σ- (v+, J+) 13. 01 e. V 3 2Π (v′, J′) 12. 1 e. V D 2Σ- (v′, J′) 10. 14 e. V 118 nm 10. 14 e. V < 307 nm 122 nm 244 nm 228 nm A 2 Σ+ (v′=1, 2, J′) 4. 73 e. V 4. 38 e. V 283 nm 262 nm X 2Π (v′′, J′′, Fi) Beames et al. , J. Chem. Phys. 134, 241102 (2011)

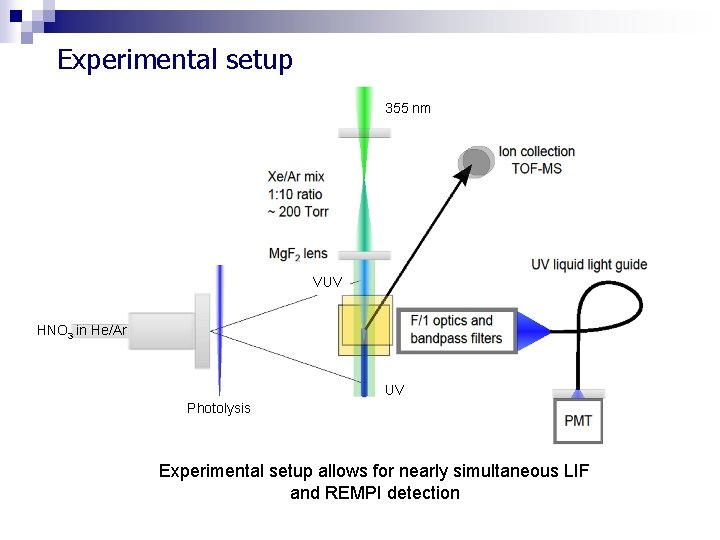
Experimental setup 355 nm VUV HNO 3 in He/Ar UV Photolysis Experimental setup allows for nearly simultaneous LIF and REMPI detection

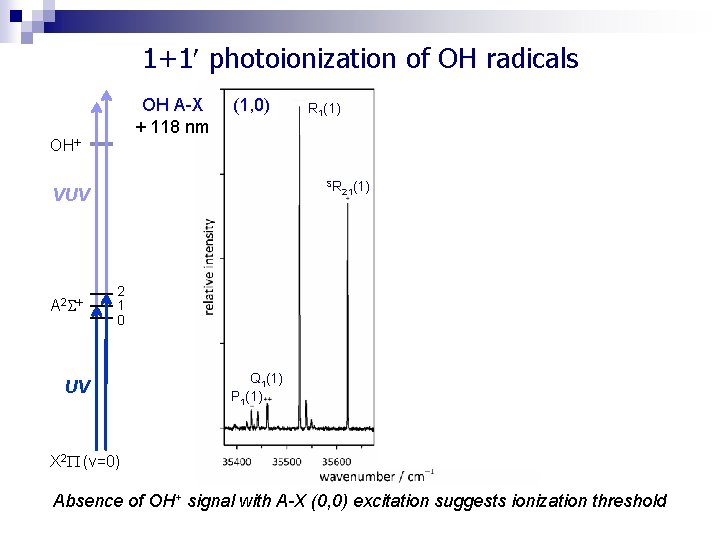
1+1 photoionization of OH radicals OH A-X + 118 nm OH (1, 0) SR VUV A 2 (2, 0) R 1(1) 21(1) SR 2 1 0 UV 21(1) Q 1(1) P 1(1) X 2 (v=0) Absence of OH+ signal with A-X (0, 0) excitation suggests ionization threshold

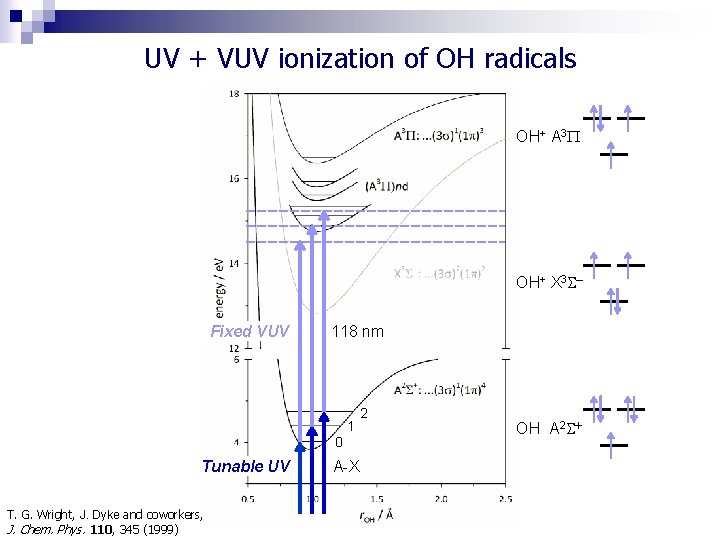
UV + VUV ionization of OH radicals OH+ A 3 OH+ X 3 – Fixed VUV 118 nm 1 0 Tunable UV T. G. Wright, J. Dyke and coworkers, J. Chem. Phys. 110, 345 (1999) A-X 2 OH A 2

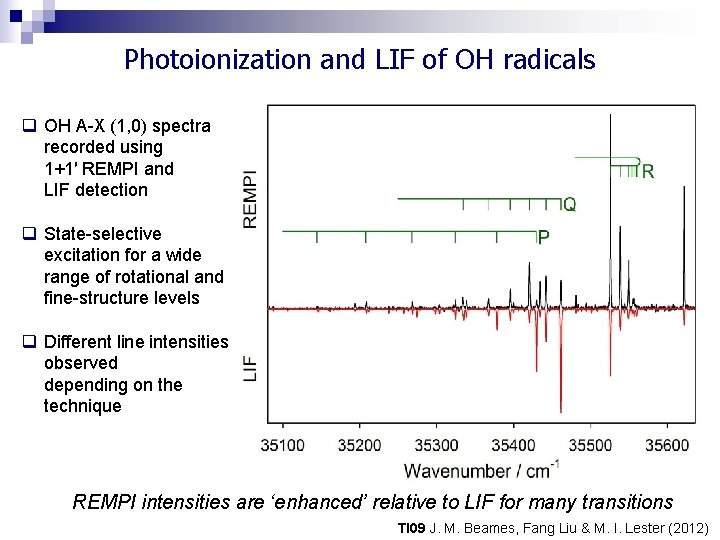
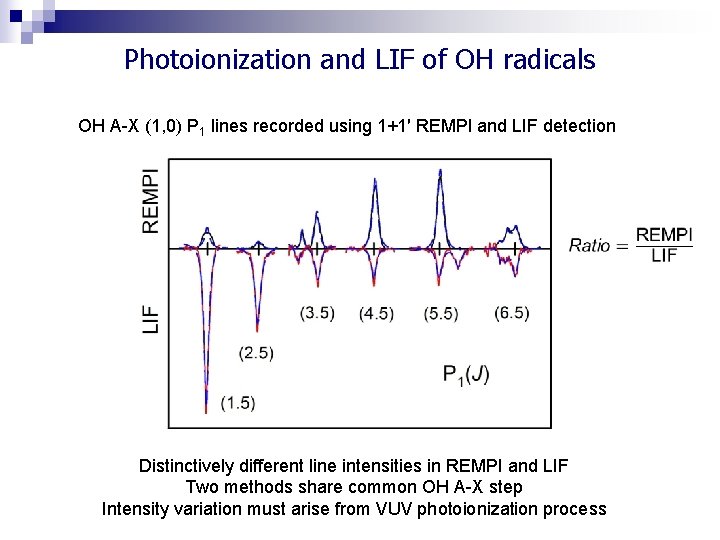
Photoionization and LIF of OH radicals q OH A-X (1, 0) spectra recorded using 1+1′ REMPI and LIF detection q State-selective excitation for a wide range of rotational and fine-structure levels q Different line intensities observed depending on the technique REMPI intensities are ‘enhanced’ relative to LIF for many transitions TI 09 J. M. Beames, Fang Liu & M. I. Lester (2012)

Photoionization and LIF of OH radicals OH A-X (1, 0) P 1 lines recorded using 1+1′ REMPI and LIF detection Distinctively different line intensities in REMPI and LIF Two methods share common OH A-X step Intensity variation must arise from VUV photoionization process

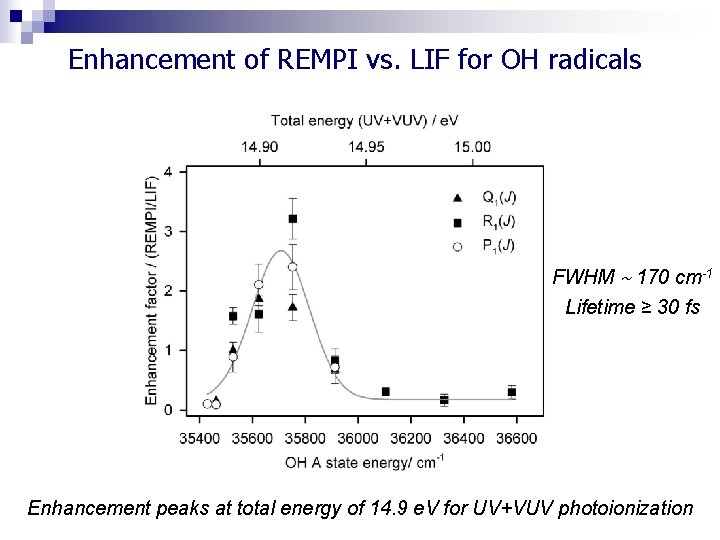
Enhancement of REMPI vs. LIF for OH radicals FWHM 170 cm-1 Lifetime ≥ 30 fs Enhancement peaks at total energy of 14. 9 e. V for UV+VUV photoionization

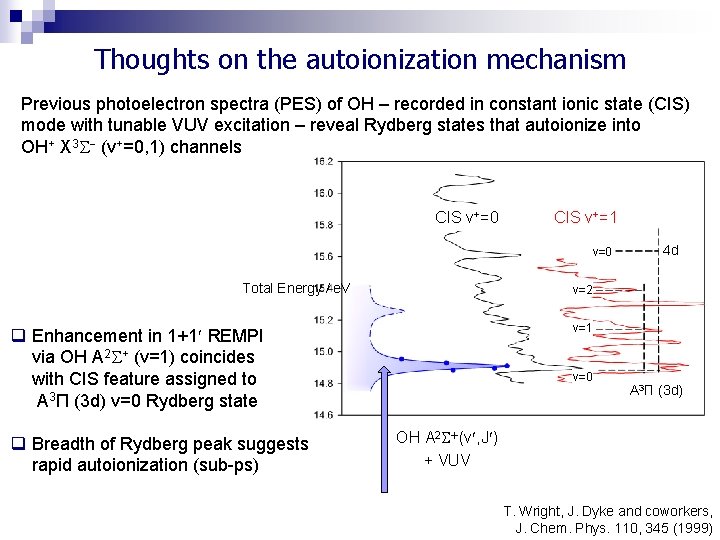
Thoughts on the autoionization mechanism Previous photoelectron spectra (PES) of OH – recorded in constant ionic state (CIS) mode with tunable VUV excitation – reveal Rydberg states that autoionize into OH+ X 3 (v+=0, 1) channels CIS v+=0 CIS v+=1 v=0 Total Energy / e. V v=2 v=1 q Enhancement in 1+1 REMPI via OH A 2 + (v=1) coincides with CIS feature assigned to A 3Π (3 d) v=0 Rydberg state q Breadth of Rydberg peak suggests rapid autoionization (sub-ps) 4 d v=0 A 3Π (3 d) OH A 2 (v , J ) + VUV T. Wright, J. Dyke and coworkers, J. Chem. Phys. 110, 345 (1999)

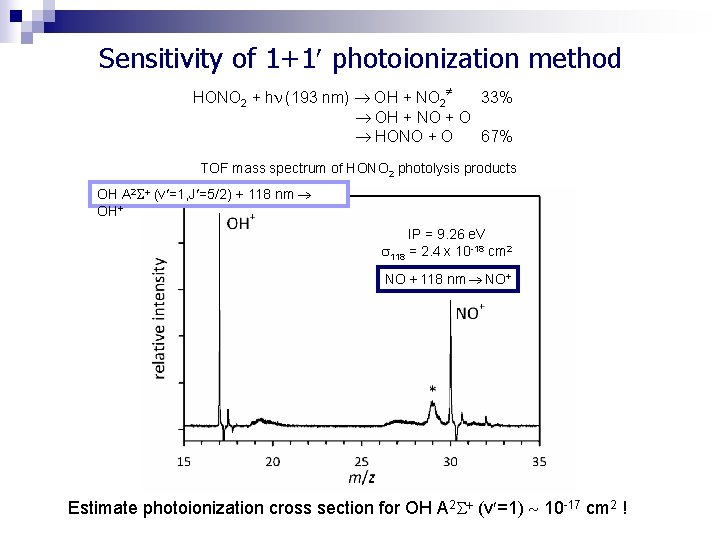
Sensitivity of 1+1 photoionization method HONO 2 + h (193 nm) OH + NO 2 33% OH + NO + O HONO + O 67% TOF mass spectrum of HONO 2 photolysis products OH A 2 (v =1, J =5/2) + 118 nm OH+ IP = 9. 26 e. V 118 = 2. 4 x 10 -18 cm 2 NO + 118 nm NO+ Estimate photoionization cross section for OH A 2 (v =1) 10 -17 cm 2 !

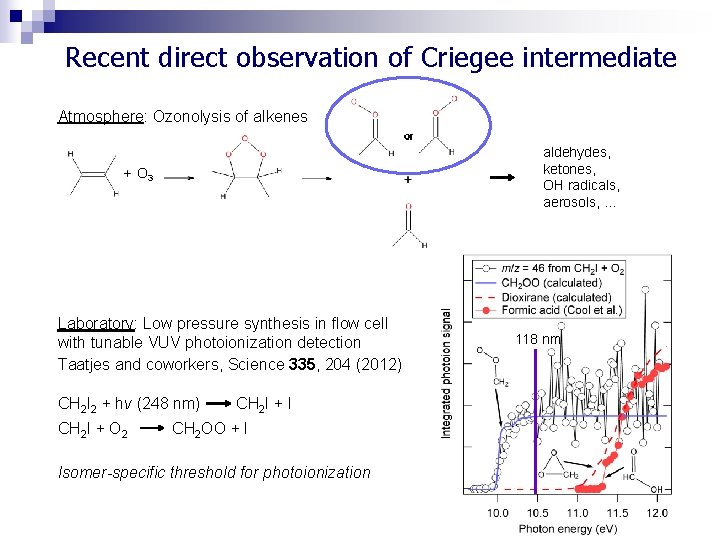
Recent direct observation of Criegee intermediate Atmosphere: Ozonolysis of alkenes aldehydes, ketones, OH radicals, aerosols, … O 3 Laboratory: Low pressure synthesis in flow cell with tunable VUV photoionization detection Taatjes and coworkers, Science 335, 204 (2012) CH 2 I 2 + hv (248 nm) CH 2 I + O 2 CH 2 I + I CH 2 OO + I Isomer-specific threshold for photoionization 118 nm

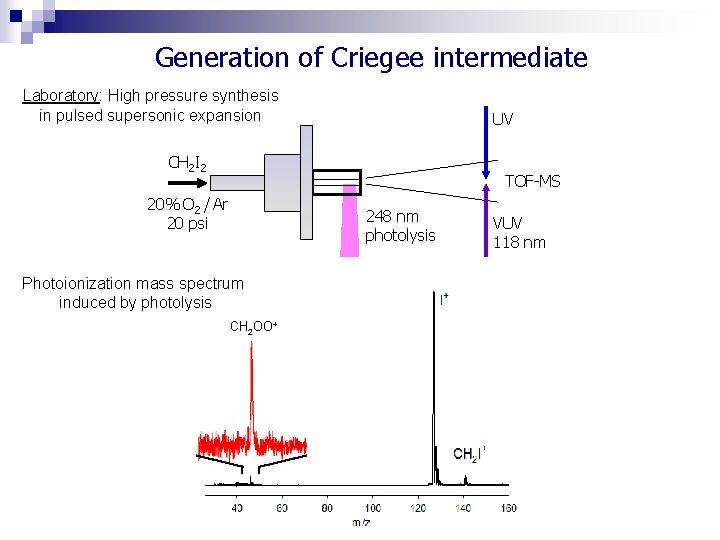
Generation of Criegee intermediate Laboratory: High pressure synthesis in pulsed supersonic expansion UV CH 2 I 2 TOF-MS 20% O 2 / Ar 20 psi 248 nm photolysis Photoionization mass spectrum induced by photolysis CH 2 OO+ VUV 118 nm

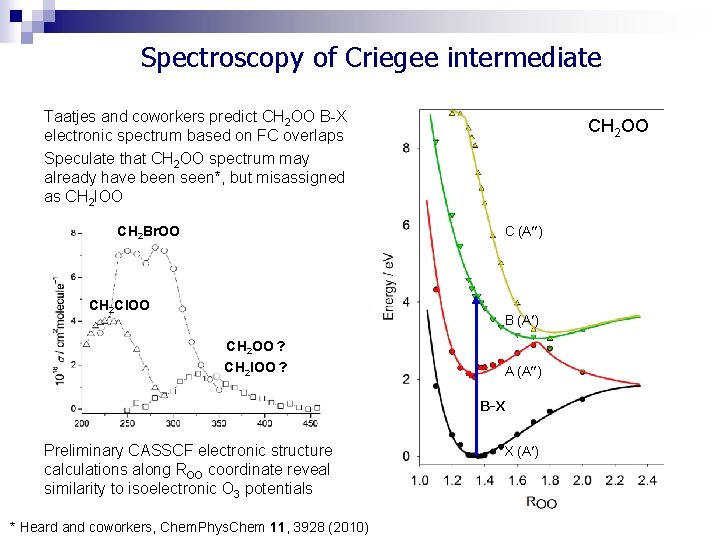
Spectroscopy of Criegee intermediate Taatjes and coworkers predict CH 2 OO B-X electronic spectrum based on FC overlaps Speculate that CH 2 OO spectrum may already have been seen*, but misassigned as CH 2 IOO CH 2 Br. OO CH 2 OO C (A ) CH 2 Cl. OO B (A ) CH 2 OO ? CH 2 IOO ? A (A ) B-X Preliminary CASSCF electronic structure calculations along ROO coordinate reveal similarity to isoelectronic O 3 potentials * Heard and coworkers, Chem. Phys. Chem 11, 3928 (2010) X (A )

Ongoing Efforts n Focus on spectroscopy and dynamics of simplest Criegee intermediate CH 2 OO and development / utilization of photoionization schemes for substituted Criegees n New 1+1 REMPI scheme via OH A 2 + (v=1) enables quantitative detection of OH X 2 (v=0 -2) by photoionization for variety of applications including HOOO n Collaborative efforts underway to measure the kinetic energy and angular distributions of the photoelectrons n Setting up tunable VUV to probe Rydberg states directly

Acknowledgements People at Penn: Tim Sechler, Julia Lehman, Craig Murray, * Logan Dempsey, MIL, Pesia Soloveichik, Bridget O’Donnell, Erika Derro [Joe Beames, Fang Liu] * Now a Lecturer at U. Glasgow