Soil Mechanics3 rd class Highway Transportation Dept Chapter



- Slides: 22

Soil Mechanics-3 rd class Highway & Transportation Dept. Chapter 1 Physico-chemical considerations of saturated clays Al-Mustansirya, Baghdad College of Engineering Highway and Transportation Department Soil Mechanics Dr. Yasir M. Al-Badran

Soil Mechanics-3 rd class Highway & Transportation Dept. Outline • Basic Unit of Clay Minerals • Interaction of Clay Particles (or Layers) • Clay Minerals: • Kaolinite • Illite • Montmorillonite • Soil Micro-structure • Particle Associations on Clay • Fabric of Natural Clay Soils • Domains and clusters with micropores • Interaction Forces between clay interlayers • Van der Waals attraction (A) • Double layer repulsion (R) Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 2

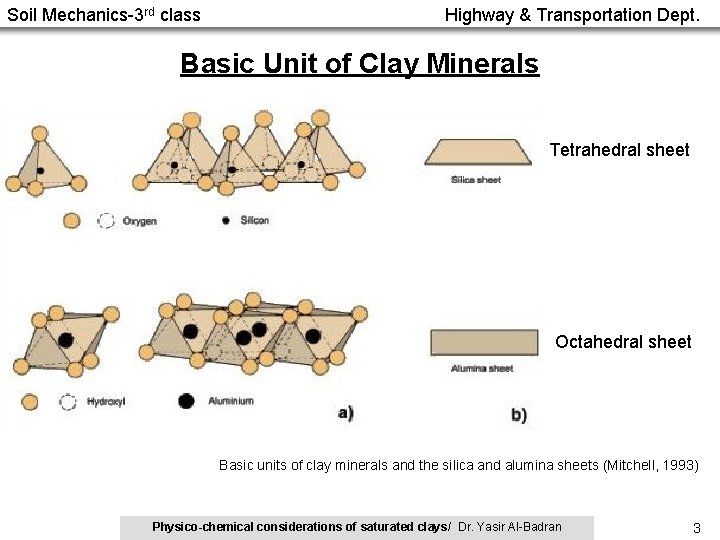
Soil Mechanics-3 rd class Highway & Transportation Dept. Basic Unit of Clay Minerals Tetrahedral sheet Octahedral sheet Basic units of clay minerals and the silica and alumina sheets (Mitchell, 1993) Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 3

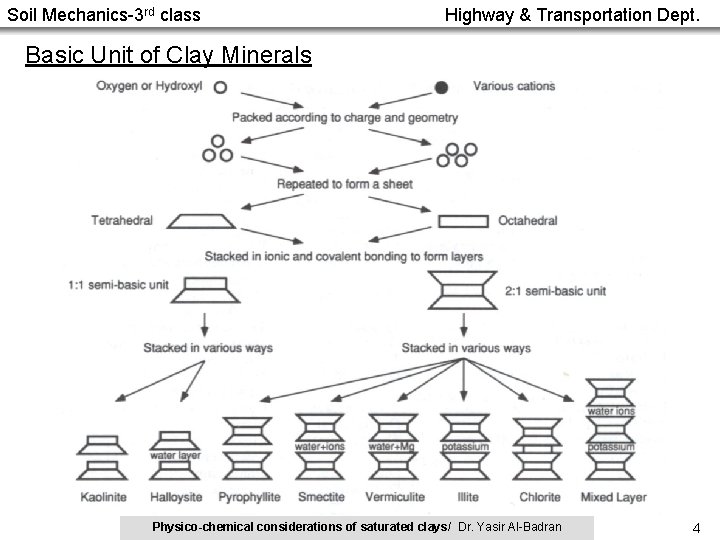
Soil Mechanics-3 rd class Highway & Transportation Dept. Basic Unit of Clay Minerals Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 4

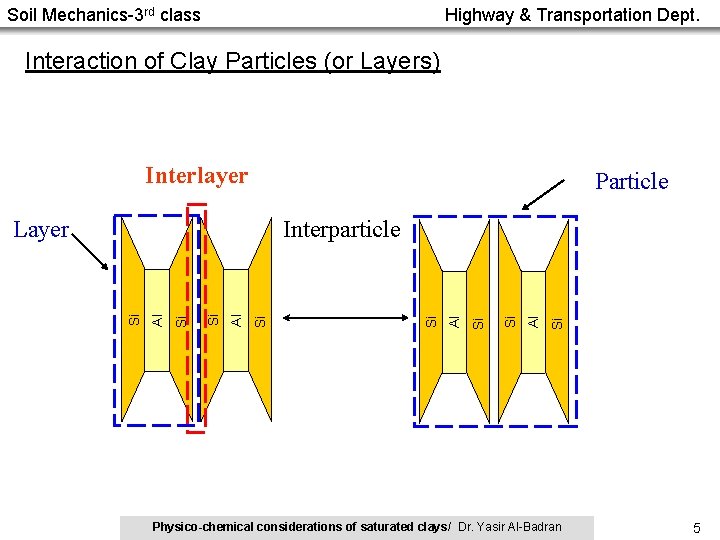
Soil Mechanics-3 rd class Highway & Transportation Dept. Interaction of Clay Particles (or Layers) Interlayer Particle Si Al Si Si Al Interparticle Si Layer Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 5

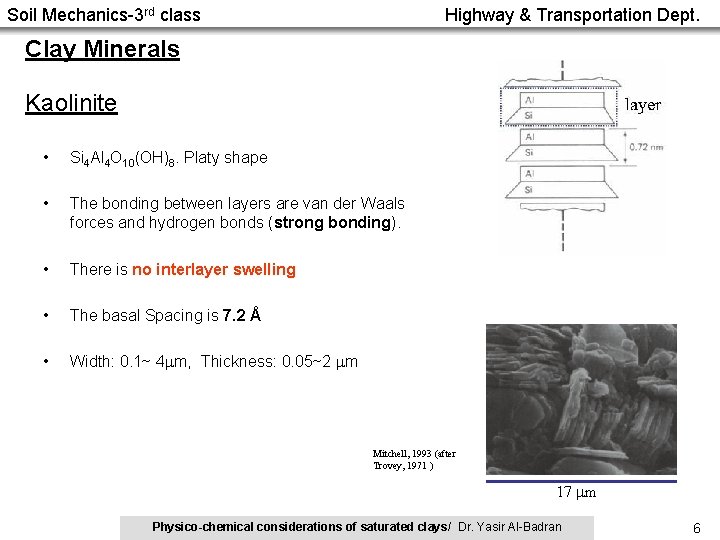
Soil Mechanics-3 rd class Highway & Transportation Dept. Clay Minerals Kaolinite • Si 4 Al 4 O 10(OH)8. Platy shape • The bonding between layers are van der Waals forces and hydrogen bonds (strong bonding). • There is no interlayer swelling • The basal Spacing is 7. 2 Å • Width: 0. 1~ 4 m, Thickness: 0. 05~2 m Mitchell, 1993 (after Trovey, 1971 ) 17 m Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 6

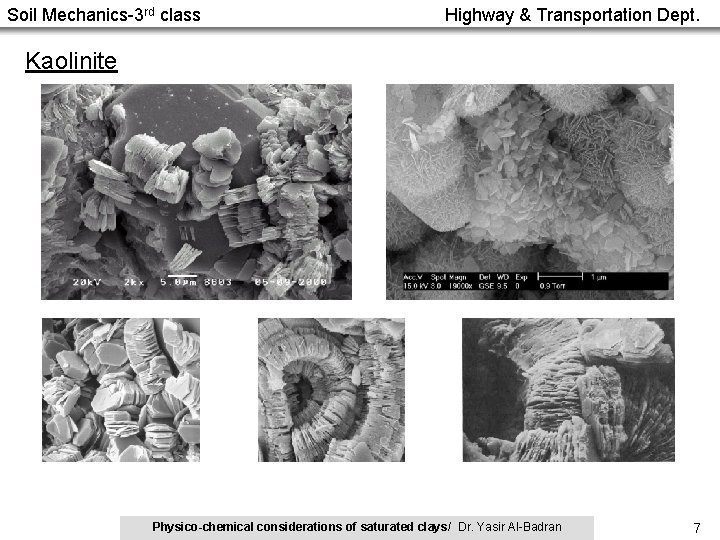
Soil Mechanics-3 rd class Highway & Transportation Dept. Kaolinite Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 7

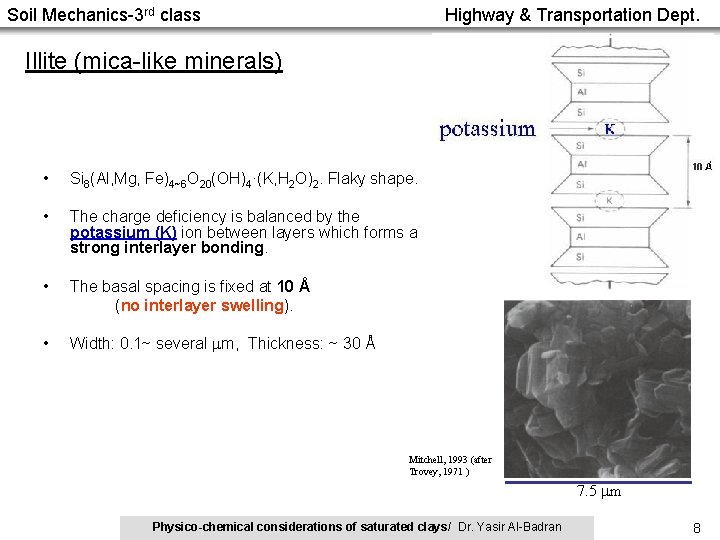
Soil Mechanics-3 rd class Highway & Transportation Dept. Illite (mica-like minerals) • Si 8(Al, Mg, Fe)4~6 O 20(OH)4·(K, H 2 O)2. Flaky shape. • The charge deficiency is balanced by the potassium (K) ion between layers which forms a strong interlayer bonding. • The basal spacing is fixed at 10 Å (no interlayer swelling). • Width: 0. 1~ several m, Thickness: ~ 30 Å Mitchell, 1993 (after Trovey, 1971 ) 7. 5 m Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 8

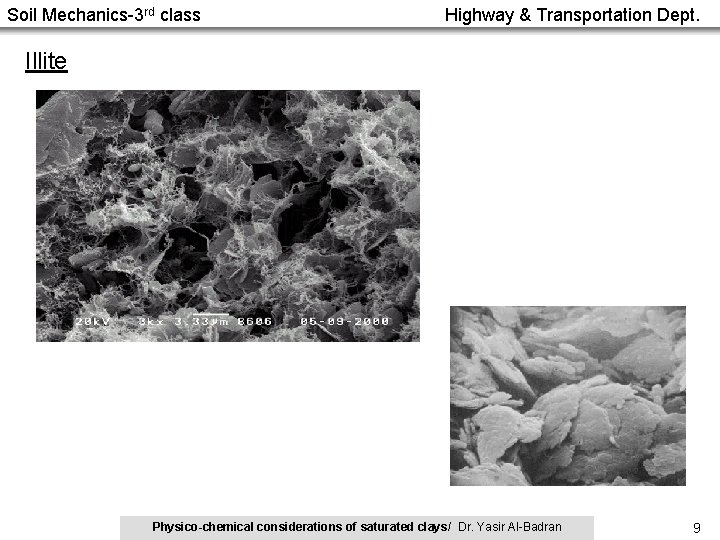
Soil Mechanics-3 rd class Highway & Transportation Dept. Illite Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 9

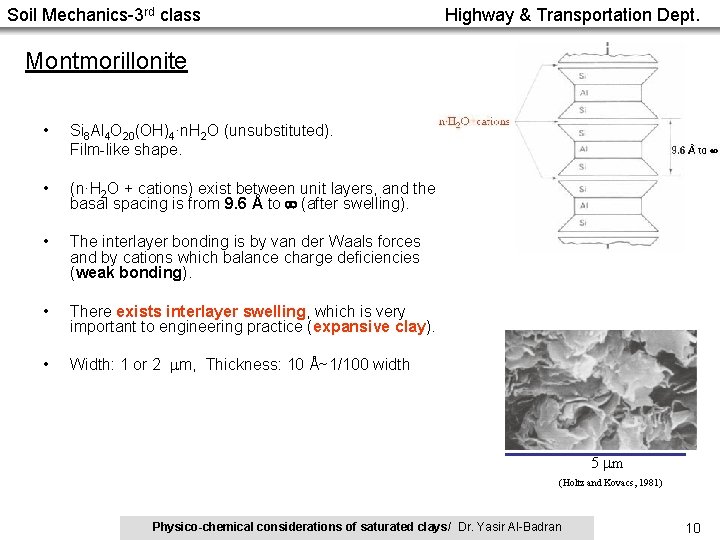
Soil Mechanics-3 rd class Highway & Transportation Dept. Montmorillonite • Si 8 Al 4 O 20(OH)4·n. H 2 O (unsubstituted). Film-like shape. • (n·H 2 O + cations) exist between unit layers, and the basal spacing is from 9. 6 Å to (after swelling). • The interlayer bonding is by van der Waals forces and by cations which balance charge deficiencies (weak bonding). • There exists interlayer swelling, which is very important to engineering practice (expansive clay). • Width: 1 or 2 m, Thickness: 10 Å~1/100 width 5 m (Holtz and Kovacs, 1981) Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 10

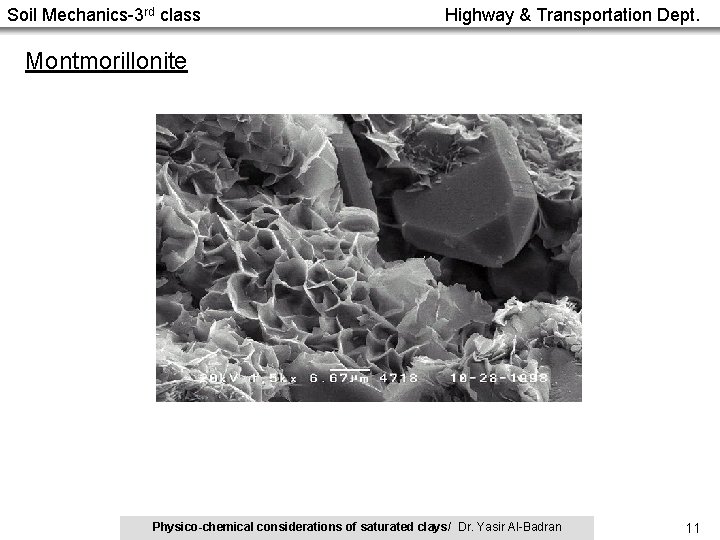
Soil Mechanics-3 rd class Highway & Transportation Dept. Montmorillonite Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 11

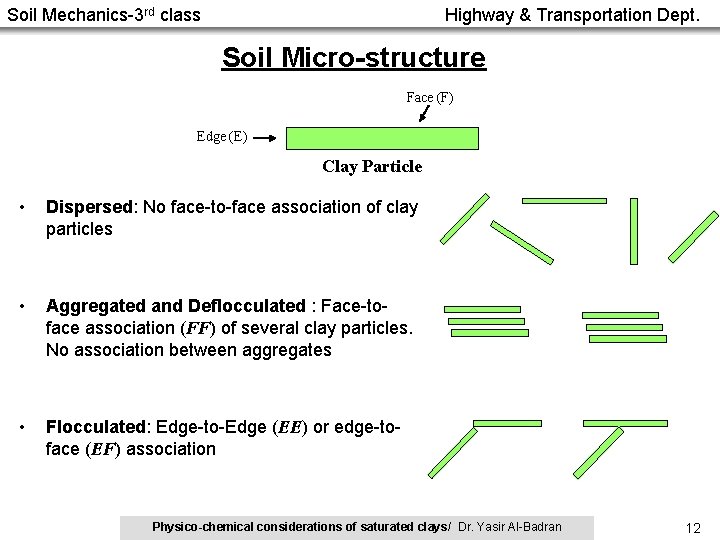
Soil Mechanics-3 rd class Highway & Transportation Dept. Soil Micro-structure Face (F) Edge (E) Clay Particle • Dispersed: No face-to-face association of clay particles • Aggregated and Deflocculated : Face-toface association (FF) of several clay particles. No association between aggregates • Flocculated: Edge-to-Edge (EE) or edge-toface (EF) association Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 12

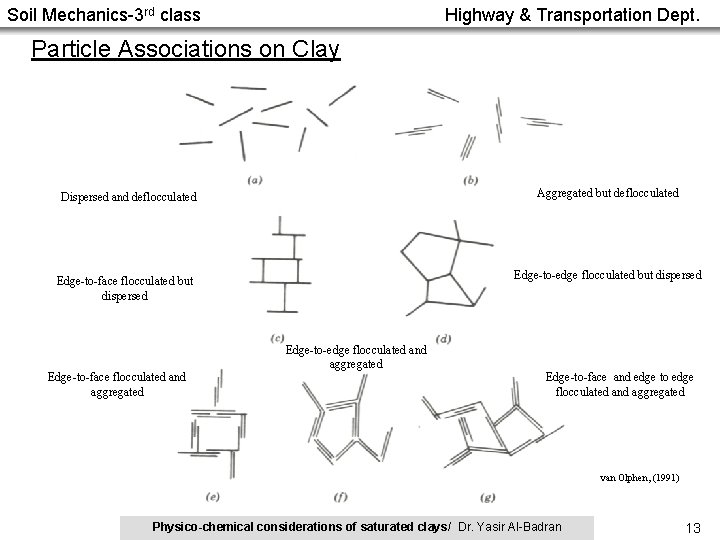
Soil Mechanics-3 rd class Highway & Transportation Dept. Particle Associations on Clay Aggregated but deflocculated Dispersed and deflocculated Edge-to-edge flocculated but dispersed Edge-to-face flocculated and aggregated Edge-to-edge flocculated and aggregated Edge-to-face and edge to edge flocculated and aggregated van Olphen, (1991) Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 13

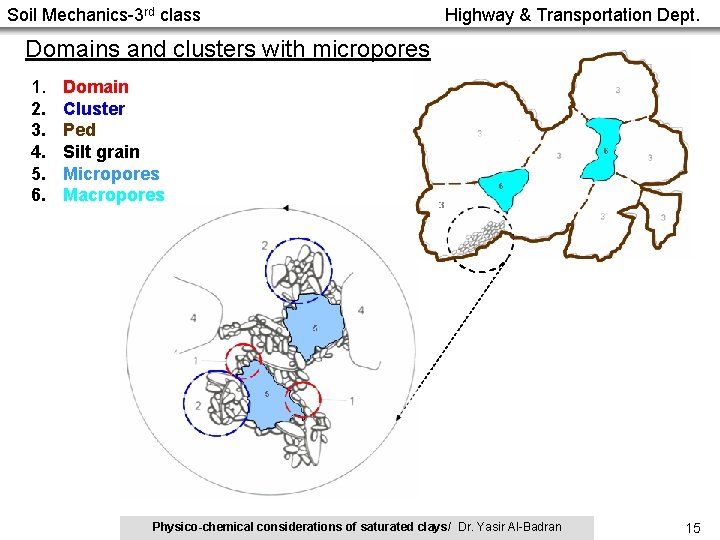
Soil Mechanics-3 rd class Highway & Transportation Dept. Fabric of Natural Clay Soils • The individual clay particles seem to always be aggregated or flocculated together in submicroscopic fabric units called domains. • Domains then in turn group together to form clusters, which are large enough to be seen with a visible light microscope. • Clusters group together to form peds and even groups of peds. Peds can be seen without a microscope, and they and other macrostructural features such as joints and fissures constitute the macrofabric system (Holtz and Kovacs, 1981). Domain Cluster Ped Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 14

Soil Mechanics-3 rd class Highway & Transportation Dept. Domains and clusters with micropores 1. 2. 3. 4. 5. 6. Domain Cluster Ped Silt grain Micropores Macropores Holtz and Kovacs, 1981 (after Yong and Sheeran, 1973) Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 15

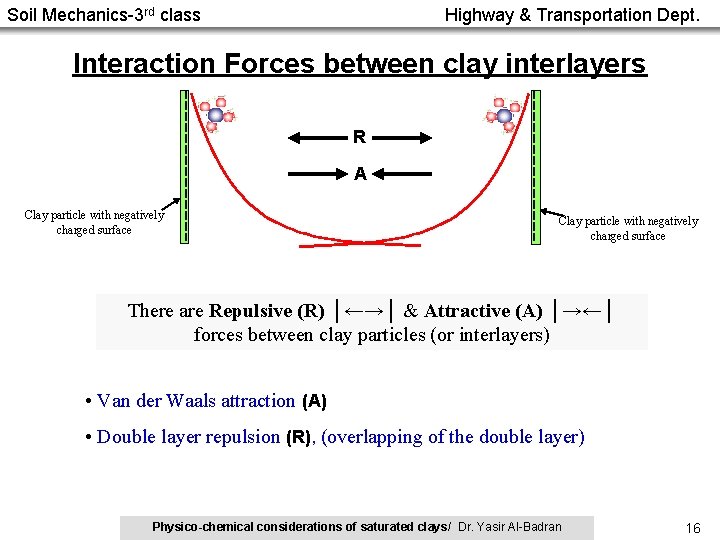
Soil Mechanics-3 rd class Highway & Transportation Dept. Interaction Forces between clay interlayers R A Clay particle with negatively charged surface There are Repulsive (R) │←→│ & Attractive (A) │→←│ forces between clay particles (or interlayers) • Van der Waals attraction (A) • Double layer repulsion (R), (overlapping of the double layer) Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 16

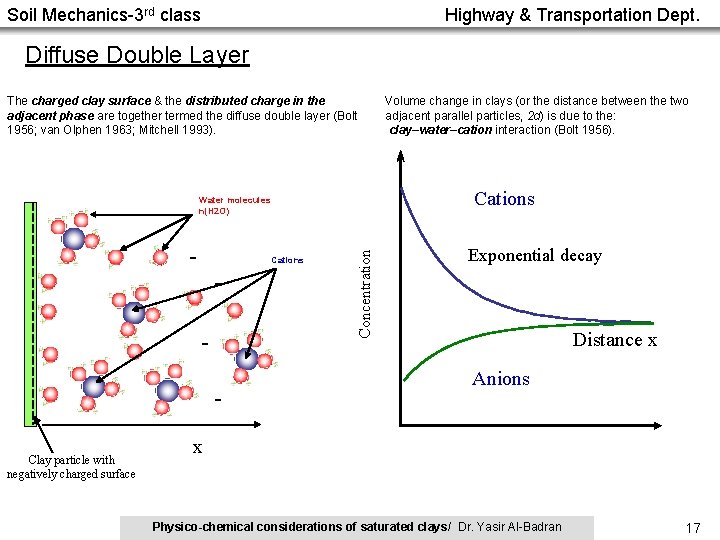
Soil Mechanics-3 rd class Highway & Transportation Dept. Diffuse Double Layer The charged clay surface & the distributed charge in the adjacent phase are together termed the diffuse double layer (Bolt 1956; van Olphen 1963; Mitchell 1993). Cations - Clay particle with negatively charged surface Concentration Water molecules n(H 2 O) - Volume change in clays (or the distance between the two adjacent parallel particles, 2 d) is due to the: clay–water–cation interaction (Bolt 1956). Exponential decay Distance x Anions x Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 17

Soil Mechanics-3 rd class Highway & Transportation Dept. Gouy-Chapman Diffuse Double Layer theory (DDL) Assumptions: • Parallel plate concept • Uniform size of clay plates • Two fluid system is considered in theory (ion concentration, n & bulk fluid concentration, n 0) • Several assumptions are made in theory, may not be true even for an ideal system: Factors NOT considered Ø Attractive pressure (e. g. van der Waals) Ø Repulsive pressure (e. g. Born repulsion) The combination of van der Waals and electrostatic forces with Born repulsion determines the nature of the overall interparticle potential (Feke et al, 1984). Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 18

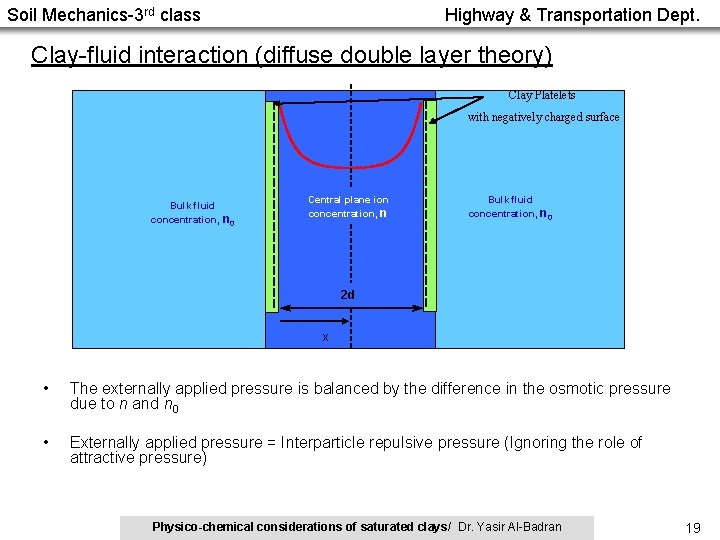
Soil Mechanics-3 rd class Highway & Transportation Dept. Clay-fluid interaction (diffuse double layer theory) Clay Platelets with negatively charged surface Bulk fluid concentration, n 0 Central plane ion concentration, n Bulk fluid concentration, n 0 2 d X • The externally applied pressure is balanced by the difference in the osmotic pressure due to n and n 0 • Externally applied pressure = Interparticle repulsive pressure (Ignoring the role of attractive pressure) Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 19

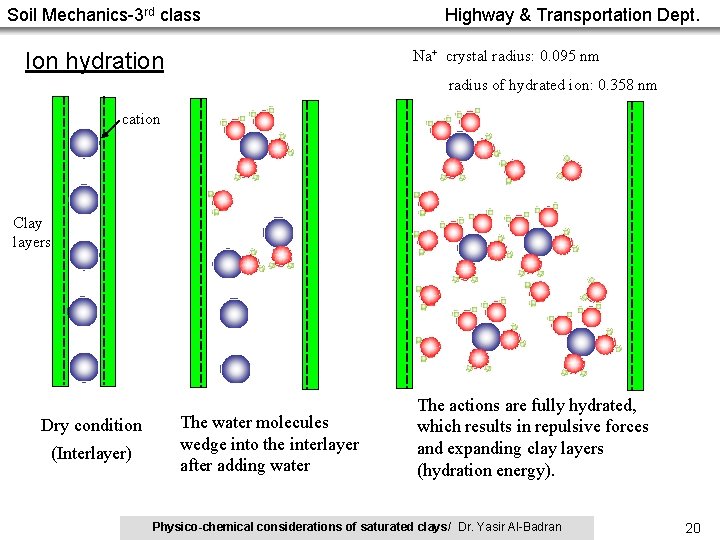
Soil Mechanics-3 rd class Highway & Transportation Dept. Na+ crystal radius: 0. 095 nm Ion hydration radius of hydrated ion: 0. 358 nm cation Clay layers Dry condition (Interlayer) The water molecules wedge into the interlayer after adding water The actions are fully hydrated, which results in repulsive forces and expanding clay layers (hydration energy). Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 20

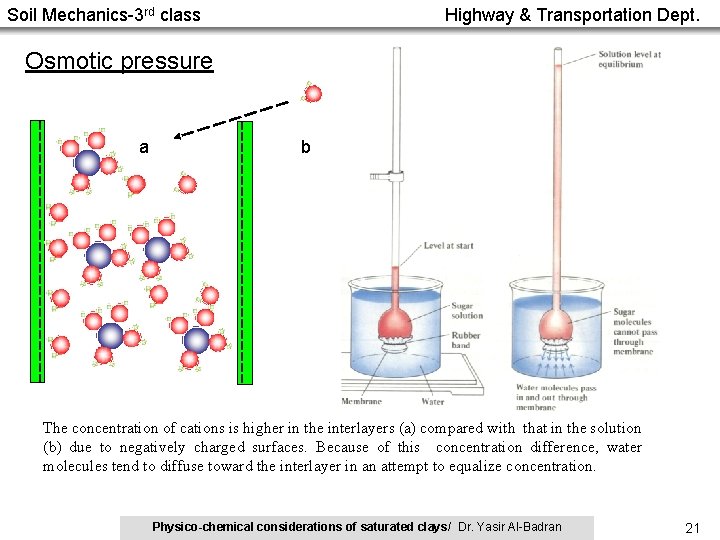
Soil Mechanics-3 rd class Highway & Transportation Dept. Osmotic pressure a b The concentration of cations is higher in the interlayers (a) compared with that in the solution (b) due to negatively charged surfaces. Because of this concentration difference, water molecules tend to diffuse toward the interlayer in an attempt to equalize concentration. Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 21

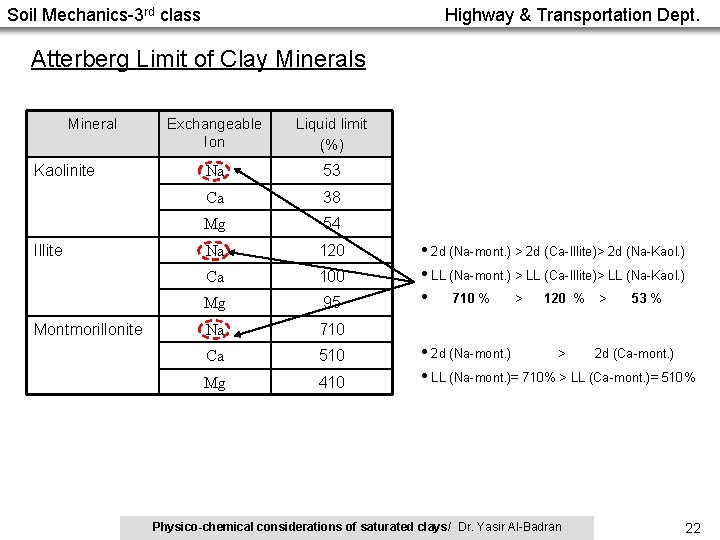
Soil Mechanics-3 rd class Highway & Transportation Dept. Atterberg Limit of Clay Minerals Mineral Kaolinite Illite Montmorillonite Exchangeable Ion Liquid limit (%) Na 53 Ca 38 Mg 54 Na 120 Ca 100 Mg 95 Na 710 Ca 510 Mg 410 • 2 d (Na-mont. ) > 2 d (Ca-Illite)> 2 d (Na-Kaol. ) • LL (Na-mont. ) > LL (Ca-Illite)> LL (Na-Kaol. ) • 710 % > 120 % > 53 % • 2 d (Na-mont. ) > 2 d (Ca-mont. ) • LL (Na-mont. )= 710% > LL (Ca-mont. )= 510% Physico-chemical considerations of saturated clays/ Dr. Yasir Al-Badran 22