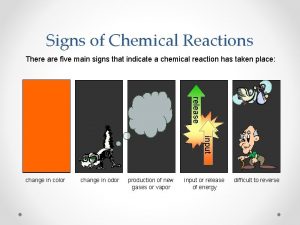
Signs of Chemical Reactions There are five main


- Slides: 13

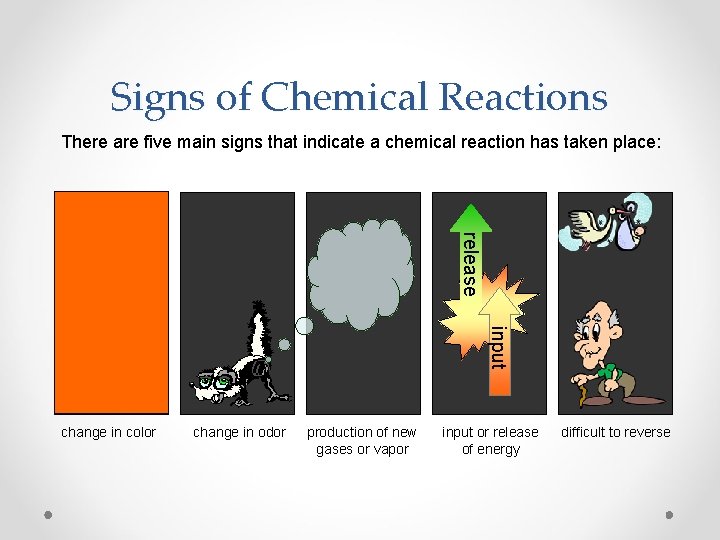
Signs of Chemical Reactions There are five main signs that indicate a chemical reaction has taken place: release input change in color change in odor production of new gases or vapor input or release of energy difficult to reverse

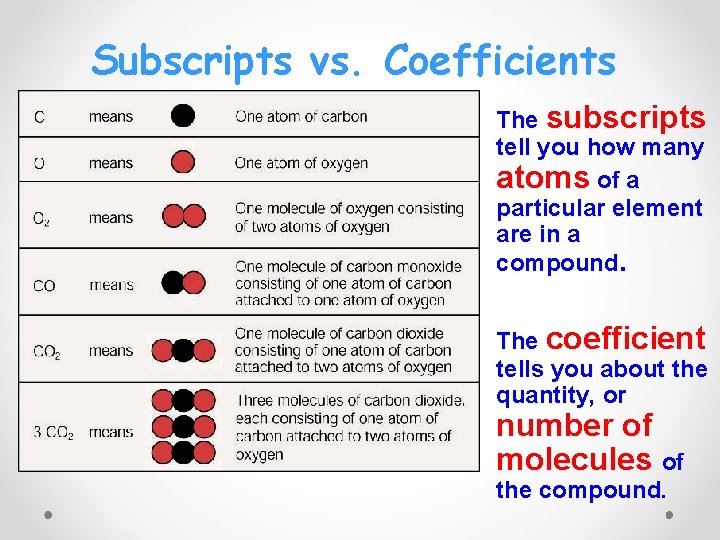
Subscripts vs. Coefficients • The subscripts tell you how many atoms of a particular element are in a compound. • The coefficient tells you about the quantity, or number of molecules of the compound.

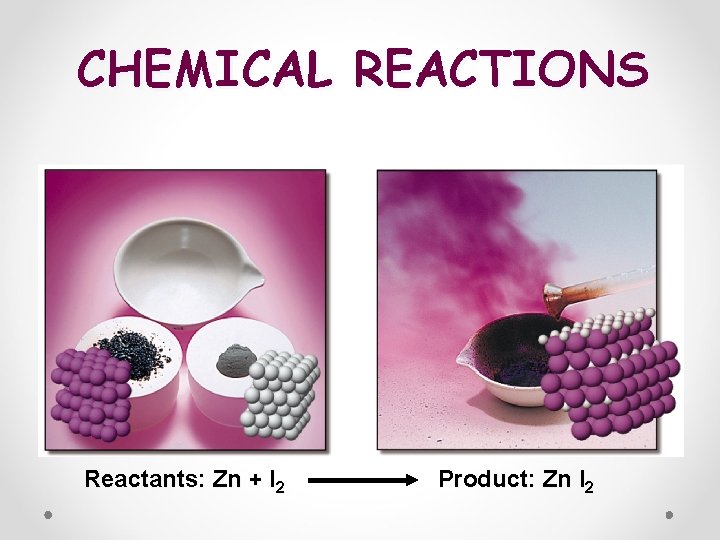
CHEMICAL REACTIONS Reactants: Zn + I 2 Product: Zn I 2

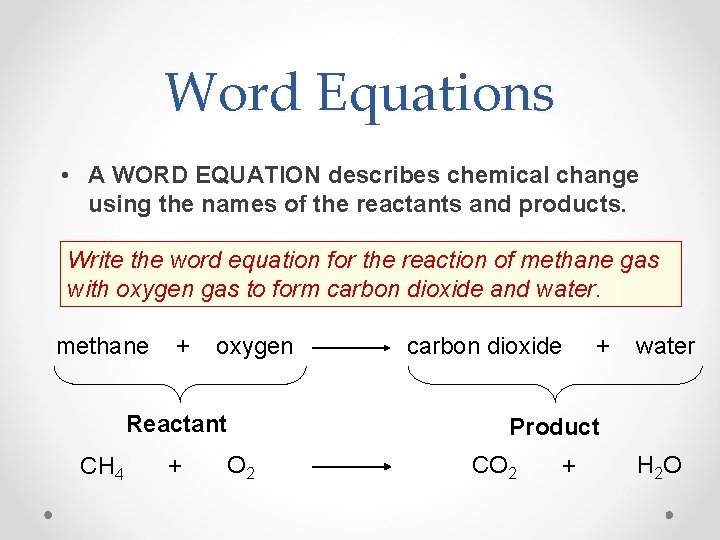
Word Equations • A WORD EQUATION describes chemical change using the names of the reactants and products. Write the word equation for the reaction of methane gas with oxygen gas to form carbon dioxide and water. methane + oxygen Reactant CH 4 + O 2 carbon dioxide + water Product CO 2 + H 2 O

Balancing Equations

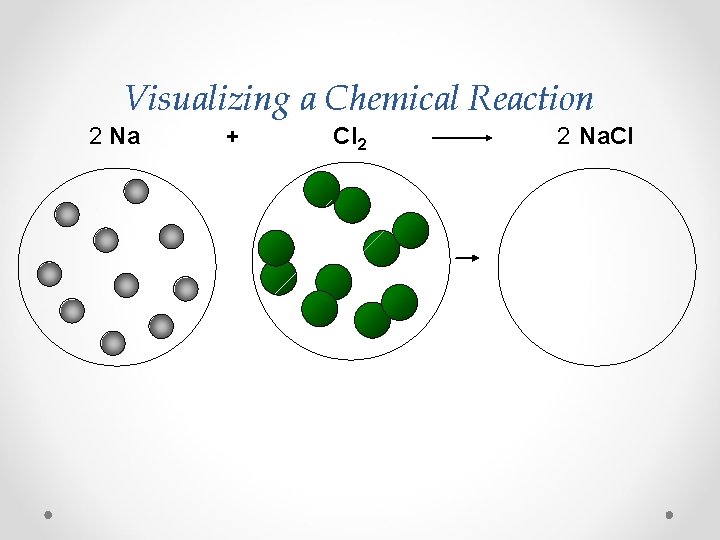
Visualizing a Chemical Reaction 2 Na + Cl 2 2 Na. Cl

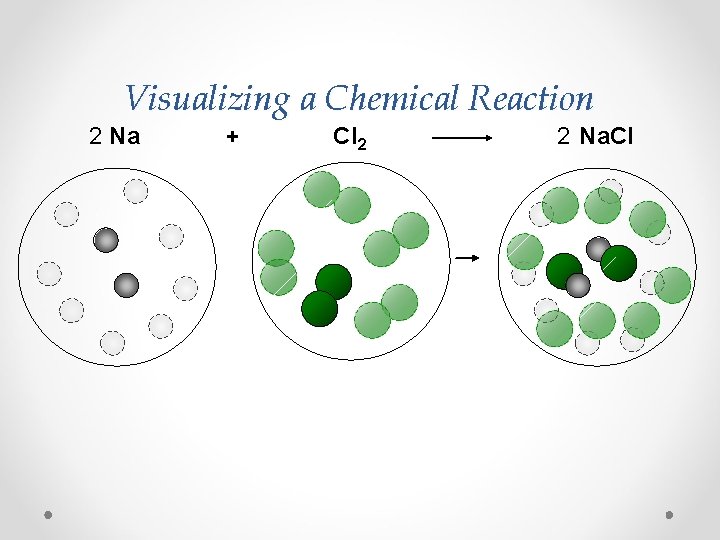
Visualizing a Chemical Reaction 2 Na + Cl 2 2 Na. Cl

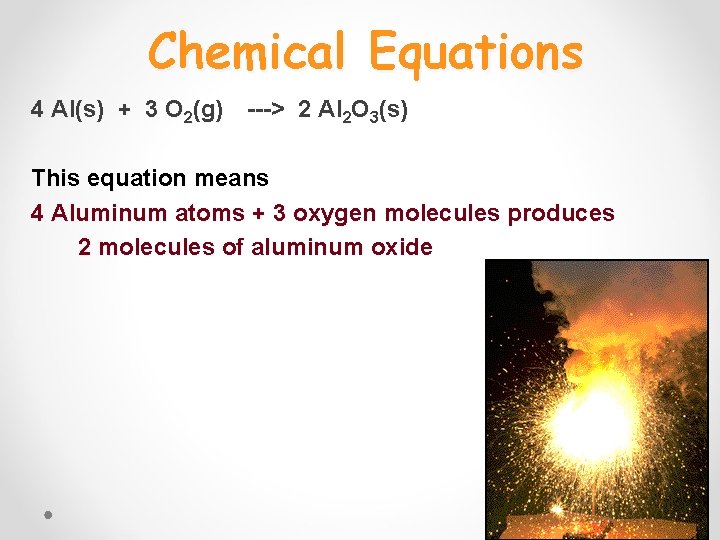
Chemical Equations 4 Al(s) + 3 O 2(g) ---> 2 Al 2 O 3(s) This equation means 4 Aluminum atoms + 3 oxygen molecules produces 2 molecules of aluminum oxide

Balancing Equations

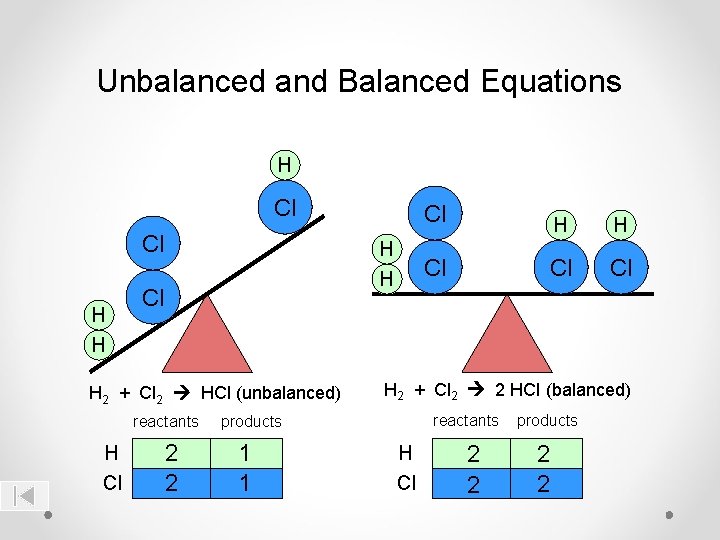
Unbalanced and Balanced Equations H Cl Cl H H Cl H 2 + Cl 2 HCl (unbalanced) reactants H Cl 2 2 H H Cl Cl Cl H 2 + Cl 2 2 HCl (balanced) reactants products 1 1 Cl H Cl 2 2 products 2 2

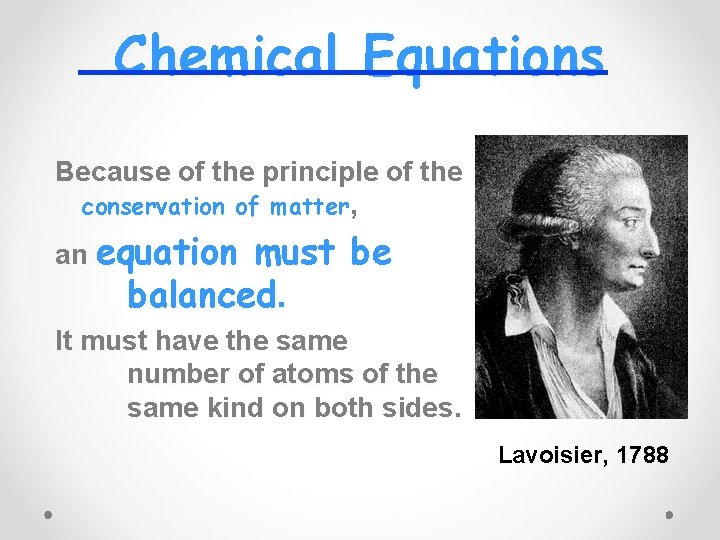
Chemical Equations Because of the principle of the conservation of matter, an equation must be balanced. It must have the same number of atoms of the same kind on both sides. Lavoisier, 1788

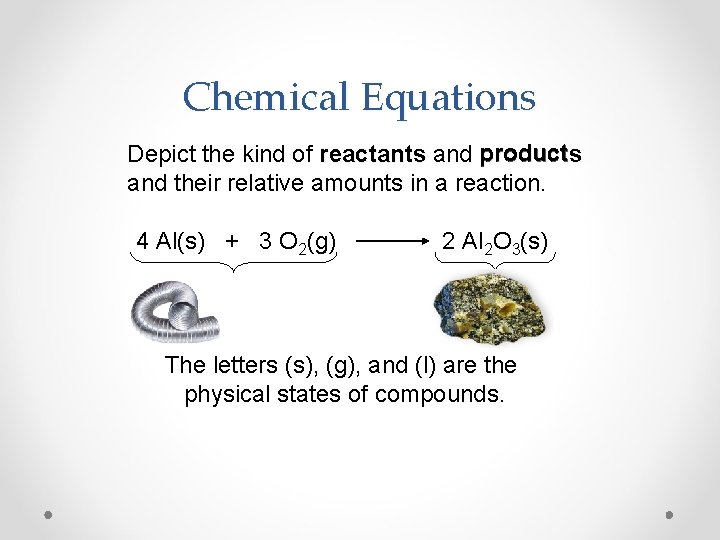
Chemical Equations Depict the kind of reactants and products and their relative amounts in a reaction. 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) The letters (s), (g), and (l) are the physical states of compounds.

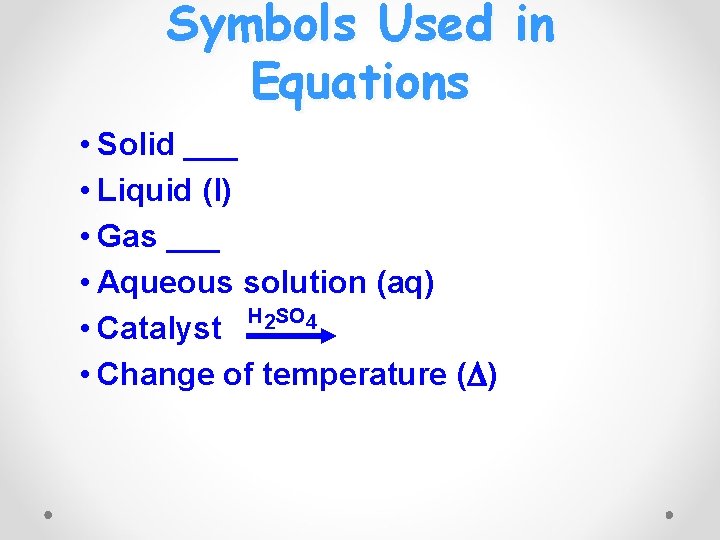
Symbols Used in Equations • Solid ___ • Liquid (l) • Gas ___ • Aqueous solution (aq) H 2 SO 4 • Catalyst • Change of temperature ( )
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Mikael ferm
Mikael ferm Types of reactions
Types of reactions Sign sign everywhere a sign
Sign sign everywhere a sign What are the five general types of chemical reactions
What are the five general types of chemical reactions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal 10 examples of redox reaction
10 examples of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Types of reactions
Types of reactions Natural and conventional signs in semantics
Natural and conventional signs in semantics The color of a recreation area sign is ______________.
The color of a recreation area sign is ______________. Triangular signs are used exclusively for _____ signs.
Triangular signs are used exclusively for _____ signs.