Shock Reduction History James Coles Ph D Medtronic





- Slides: 10

Shock Reduction History James Coles, Ph. D Medtronic, Inc.

Avoiding Shocks Is Important To Reduce Pain and Anxiety and Increase Device Acceptance 1 To Reduce Healthcare Burden and Improve Patient Quality of Life 1 Avoiding Shocks May Improve Survival/Heart Failure 2 1 Wathen MS, De. Groot PJ, Sweeney MO, et al, for the Pain. FREE Rx II Investigators. Prospective randomized multicenter trial of empirical antitachycardia pacing versus shocks for spontaneous rapid ventricular tachycardia in patients with implantable cardioverter-defibrillators: Pacing Fast Ventricular Tachycardia Reduces Shock Therapies (Pain. FREE Rx II) trial results. Circulation. October 26, 2004; 110(17): 2591 -2596. 2 Sweeney MO, et al. Differences in effects of electrical therapy type on health care utilization in the MVP ICD Trial. Presented at HRS 2010.

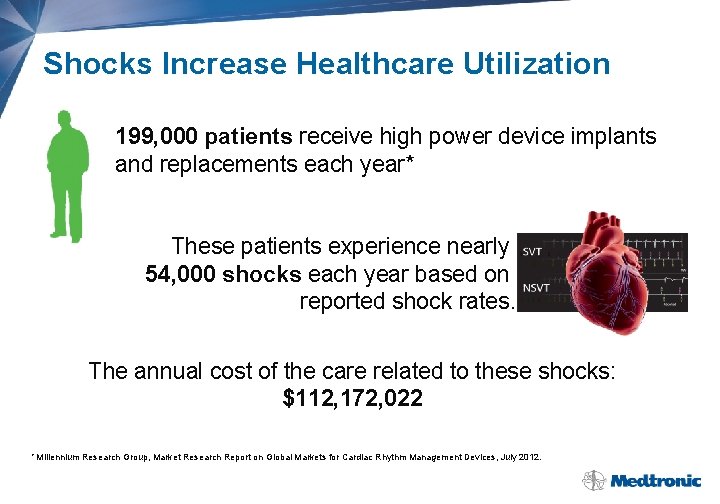
Shocks Increase Healthcare Utilization 199, 000 patients receive high power device implants and replacements each year* These patients experience nearly 54, 000 shocks each year based on reported shock rates. The annual cost of the care related to these shocks: $112, 172, 022 * Millennium Research Group, Market Research Report on Global Markets for Cardiac Rhythm Management Devices, July 2012.

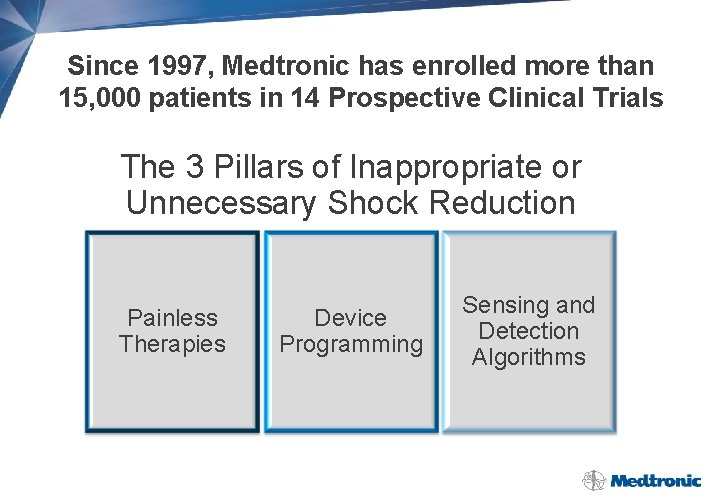
Since 1997, Medtronic has enrolled more than 15, 000 patients in 14 Prospective Clinical Trials The 3 Pillars of Inappropriate or Unnecessary Shock Reduction Painless Therapies Device Programming Sensing and Detection Algorithms

Painless Therapies: The Evolution of Anti-Tachycardia Pacing (ATP) in ICDs Safe and Effective Pain. Free I 1 & II 2 (n = 854) • 76% of device detected VF is actually Fast VT • ATP successfully terminated 3 out of 4 Fast VTs • ATP added no additional risk from syncope or acceleration Applies to all Devices ADVANCE CRT-D 4 (n = 526) • A single burst of ATP effectively and safely terminates 68% of VT in CRT-D patients • No differences in ATP efficacy between Bi. V and RV-delivered ATP Nominally “On" En. Trust 5 (n= 421) • ATP during charging can terminate 69% of Fast VT with low a risk of acceleration or symptoms

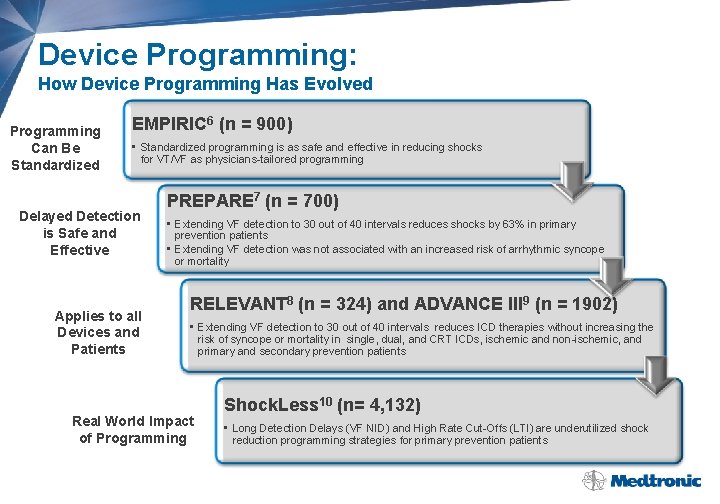
Device Programming: How Device Programming Has Evolved Programming Can Be Standardized EMPIRIC 6 (n = 900) • Standardized programming is as safe and effective in reducing shocks for VT/VF as physicians-tailored programming Delayed Detection is Safe and Effective Applies to all Devices and Patients PREPARE 7 (n = 700) • Extending VF detection to 30 out of 40 intervals reduces shocks by 63% in primary prevention patients • Extending VF detection was not associated with an increased risk of arrhythmic syncope or mortality RELEVANT 8 (n = 324) and ADVANCE III 9 (n = 1902) • Extending VF detection to 30 out of 40 intervals reduces ICD therapies without increasing the risk of syncope or mortality in single, dual, and CRT ICDs, ischemic and non-ischemic, and primary and secondary prevention patients Real World Impact of Programming Shock. Less 10 (n= 4, 132) • Long Detection Delays (VF NID) and High Rate Cut-Offs (LTI) are underutilized shock reduction programming strategies for primary prevention patients

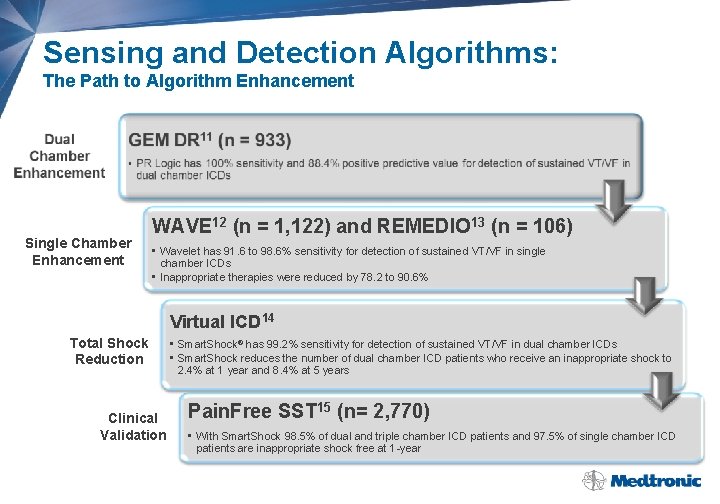
Sensing and Detection Algorithms: The Path to Algorithm Enhancement Single Chamber Enhancement WAVE 12 (n = 1, 122) and REMEDIO 13 (n = 106) • Wavelet has 91. 6 to 98. 6% sensitivity for detection of sustained VT/VF in single chamber ICDs • Inappropriate therapies were reduced by 78. 2 to 90. 6% Virtual ICD 14 Total Shock Reduction Clinical Validation • Smart. Shock® has 99. 2% sensitivity for detection of sustained VT/VF in dual chamber ICDs • Smart. Shock reduces the number of dual chamber ICD patients who receive an inappropriate shock to 2. 4% at 1 year and 8. 4% at 5 years Pain. Free SST 15 (n= 2, 770) • With Smart. Shock 98. 5% of dual and triple chamber ICD patients and 97. 5% of single chamber ICD patients are inappropriate shock free at 1 -year

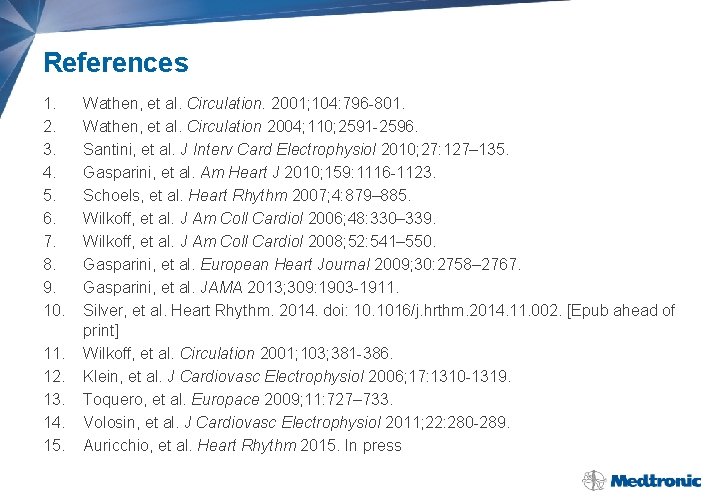
References 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. Wathen, et al. Circulation. 2001; 104: 796 -801. Wathen, et al. Circulation 2004; 110; 2591 -2596. Santini, et al. J Interv Card Electrophysiol 2010; 27: 127– 135. Gasparini, et al. Am Heart J 2010; 159: 1116 -1123. Schoels, et al. Heart Rhythm 2007; 4: 879– 885. Wilkoff, et al. J Am Coll Cardiol 2006; 48: 330– 339. Wilkoff, et al. J Am Coll Cardiol 2008; 52: 541– 550. Gasparini, et al. European Heart Journal 2009; 30: 2758– 2767. Gasparini, et al. JAMA 2013; 309: 1903 -1911. Silver, et al. Heart Rhythm. 2014. doi: 10. 1016/j. hrthm. 2014. 11. 002. [Epub ahead of print] Wilkoff, et al. Circulation 2001; 103; 381 -386. Klein, et al. J Cardiovasc Electrophysiol 2006; 17: 1310 -1319. Toquero, et al. Europace 2009; 11: 727– 733. Volosin, et al. J Cardiovasc Electrophysiol 2011; 22: 280 -289. Auricchio, et al. Heart Rhythm 2015. In press

Brief Statement: ICDs Last Updated: Oct 2014 Indications Implantable cardioverter defibrillators (ICDs) are indicated for ventricular antitachycardia pacing and ventricular defibrillation for automated treatment of life-threatening ventricular arrhythmias. Some ICDs are also indicated for use in patients with atrial tachyarrhythmias, or those patients who are at significant risk for developing atrial tachyarrhythmias. The RV Lead Integrity Alert (LIA) feature is intended primarily for patients who have a Medtronic ICD or CRT-D device and a Sprint Fidelis lead (Models 6949, 6948, 6931, and 6930, based on performance data. The RV LIA feature may not perform as well with a St. Jude Riata/Durata lead or a Boston Scientific Endotak lead as it does when used with a Medtronic Sprint Fidelis lead. This is because different lead designs may have different failure signatures and conditions that may or may not be detected early by the RV LIA feature. Notes for ICDs: The ICD features of the device functions the same as other approved Medtronic market-released ICDs. Due to the addition of the Opti. Vol diagnostic feature, the device indications are limited to the NYHA functional class II/III heart failure patients who are indicated for an ICD. The clinical value of the Opti. Vol fluid monitoring diagnostic feature has not been assessed in those patients who do not have fluid retention related symptoms due to heart failure. Additional notes for DR ICDs: The use of the device has not been demonstrated to decrease the morbidity related to atrial tachyarrhythmias. The effectiveness of high-frequency burst pacing (atrial 50 Hz Burst therapy) in terminating device classified atrial tachycardia (AT) was found to be 17%, and in terminating device classified atrial fibrillation (AF) was found to be 16. 8%, in the VT/AT patient population studied. The effectiveness of high-frequency burst pacing (atrial 50 Hz Burst therapy) in terminating device classified atrial tachycardia (AT) was found to be 11. 7%, and in terminating device classified atrial fibrillation (AF) was found to be 18. 2% in the AF-only patient population studied. Contraindications ICDs are contraindicated in patients experiencing tachyarrhythmias with transient or reversible causes including, but not limited to, the following: acute myocardial infarction, drug intoxication, drowning, electric shock, electrolyte imbalance, hypoxia, or sepsis; patients who have a unipolar pacemaker implanted, patients with incessant ventricular tachycardia (VT) or ventricular fibrillation (VF), and patients whose primary disorder is chronic atrial tachyarrhythmia with no concomitant VT or VF.

Brief Statement: ICDs Last Updated: Oct 2014 Warnings/Precautions Changes in a patient’s disease and/or medications may alter the efficacy of the device’s programmed parameters. Patients should avoid sources of magnetic and electromagnetic radiation to avoid possible underdetection, inappropriate sensing and/or therapy delivery, tissue damage, induction of an arrhythmia, device electrical reset or device damage. Do not place transthoracic defibrillation paddles directly over the device. Potential complications include, but are not limited to, rejection phenomena, erosion through the skin, muscle or nerve stimulation, oversensing, failure to detect and/or terminate arrhythmia episodes, and surgical complications such as hematoma, infection, inflammation, and thrombosis. An additional complication for ICDs is the acceleration of ventricular tachycardia. See the device manual for detailed information regarding the implant procedure, indications, contraindications, warnings, precautions, and potential complications/adverse events. For further information, please call Medtronic at 1 -800 -328 -2518 and/or consult Medtronic’s website at www. medtronic. com. Caution: Federal law (USA) restricts these devices to sale by or on the order of a physician.