Section 3 Chemical Reactions and Energy Essential Questions



- Slides: 9

Section 3 Chemical Reactions and Energy

Essential Questions How can the source of energy changes in chemical reactions be identified? How do exergonic and endergonic reactions compare? How do exothermic and endothermic reactions compare? Is energy conserved during a chemical reaction?

Review Vocabulary chemical bond: the force that holds two atoms together

New Vocabulary exergonic reaction exothermic reaction endergonic reaction endothermic reaction

Chemical Reactions—Energy Exchanges Most chemical reactions proceed more slowly, but all chemical reactions release or absorb energy. This energy can take many forms, such as thermal energy, light, sound, or electricity. The thermal energy produced by a wood fire and the light emitted by a glow stick are examples of energy released by chemical reactions.

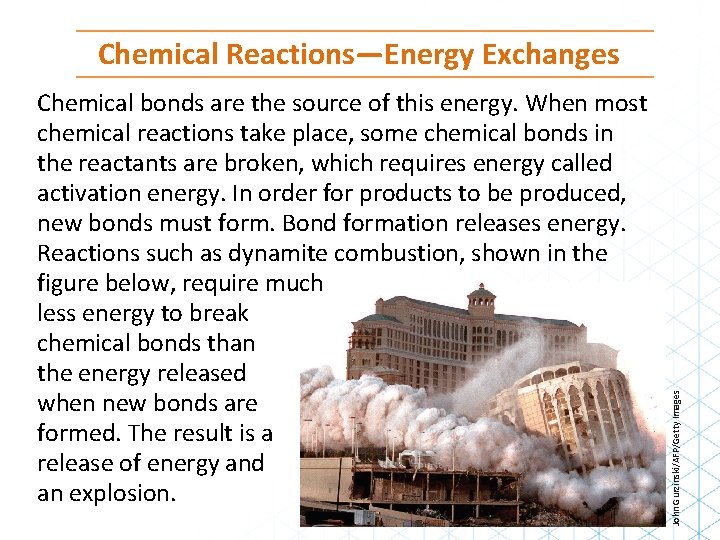
Chemical bonds are the source of this energy. When most chemical reactions take place, some chemical bonds in the reactants are broken, which requires energy called activation energy. In order for products to be produced, new bonds must form. Bond formation releases energy. Reactions such as dynamite combustion, shown in the figure below, require much less energy to break chemical bonds than the energy released when new bonds are formed. The result is a release of energy and an explosion. John Gurzinski/AFP/Getty Images Chemical Reactions—Energy Exchanges

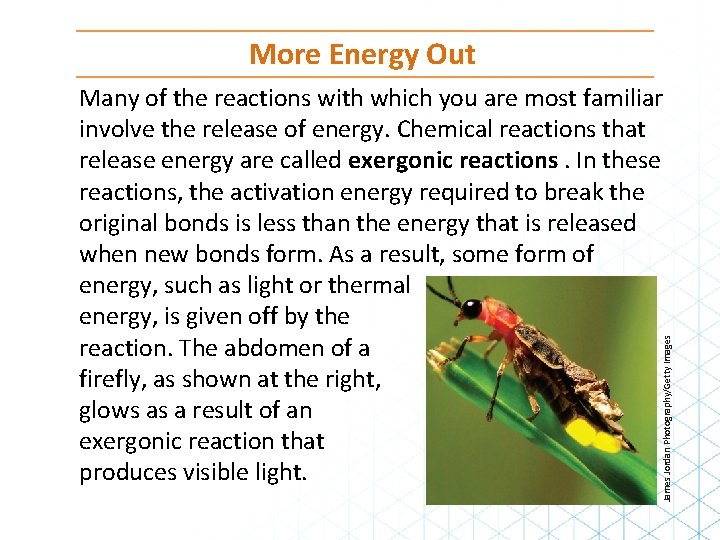
More Energy Out James Jordan Photography/Getty Images Many of the reactions with which you are most familiar involve the release of energy. Chemical reactions that release energy are called exergonic reactions. In these reactions, the activation energy required to break the original bonds is less than the energy that is released when new bonds form. As a result, some form of energy, such as light or thermal energy, is given off by the reaction. The abdomen of a firefly, as shown at the right, glows as a result of an exergonic reaction that produces visible light.

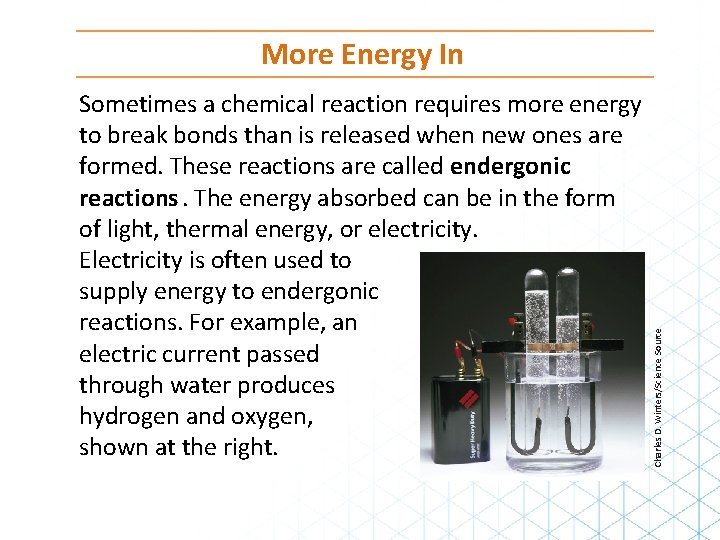
Sometimes a chemical reaction requires more energy to break bonds than is released when new ones are formed. These reactions are called endergonic reactions. The energy absorbed can be in the form of light, thermal energy, or electricity. Electricity is often used to supply energy to endergonic reactions. For example, an electric current passed through water produces hydrogen and oxygen, shown at the right. Charles D. Winters/Science Source More Energy In

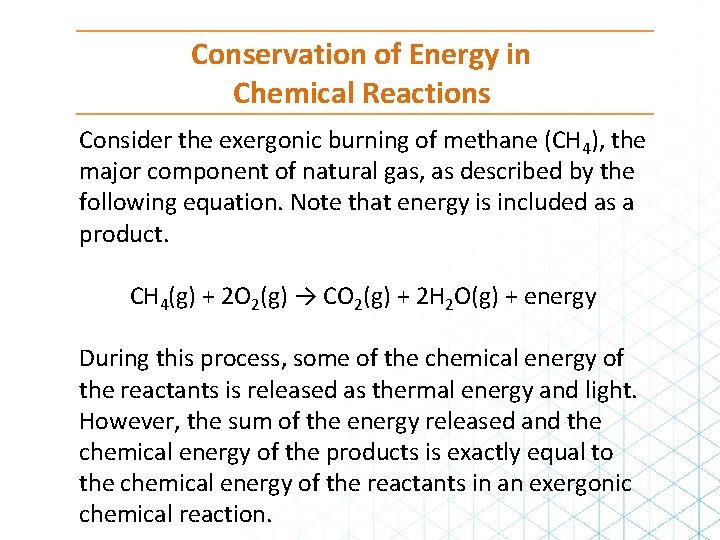
Conservation of Energy in Chemical Reactions Consider the exergonic burning of methane (CH 4), the major component of natural gas, as described by the following equation. Note that energy is included as a product. CH 4(g) + 2 O 2(g) → CO 2(g) + 2 H 2 O(g) + energy During this process, some of the chemical energy of the reactants is released as thermal energy and light. However, the sum of the energy released and the chemical energy of the products is exactly equal to the chemical energy of the reactants in an exergonic chemical reaction.
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Are kc and kp equal
Are kc and kp equal Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes What is released or absorbed whenever chemical
What is released or absorbed whenever chemical Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes

















