Role of Lipotoxicity and Gut Liver Axis in












- Slides: 22

Role of Lipotoxicity and Gut -Liver Axis in NASH Pathogenesis Dr. Perihan Salem Assistant Professor of Hepatology

�NAFLD is the accumulation of different lipid species within hepatocytes. Its spectrum includes simple steatosis and NASH. �NASH is NAFLD with hepatomegaly; abnormal ALT; fatty liver histology; lobular hepatitis and fibrosis similar to alcoholic hepatitis in the absence of alcohol intake, usually associated with obesity and DM. �Prevalence of NAFLD is 20 -40% among general population, NASH is 10 -20% among NAFLD subjects.

Lipotoxicity: *Definition of lipotoxicity. *Mechanisms of cell damage by lipotoxicity. *Toxic lipid species.

Definition of lipotoxicity: �Basically lipids are part of the cell structure and they are involved in fundamental functions as cellular homeostasis, cell-cell communication and regulation of inflammation and immunity. �Lipotoxicity is defined as dysregulation of the lipid environment or intracellular lipid composition leading to accumulation of harmful lipids (toxic lipids). �The condition started by saturated storage capacity of the adipose tissue resulting in over spilled fat to non-adipose tissues (liver). This over spilled fat stored in the form of inert TG, but the limited TG buffer capacity becomes saturated soon. Under these conditions, excess of lipids enter alternative non-oxidative pathways that result in production of harmful toxic lipid species that induce toxic responses leading to cell injury and apoptosis.

Mechanisms of cell damage by lipotoxicity: � Dysfunction of cell organelles (ER, Mitochondria) leading to cell injury and death. � Modification of intracellular signaling pathways. � Interaction between toxic lipids & cellular kinases (e. g. palmitate and TLR 4 which promotes inflammation). � Non-coding RNAs (sno. RNA), which mediate cellular responses following exposure to lipotoxic agents. Thus, hepatocellular inflammation, damage, fibrosis and cirrhosis are usually the consequences of lipotoxicity

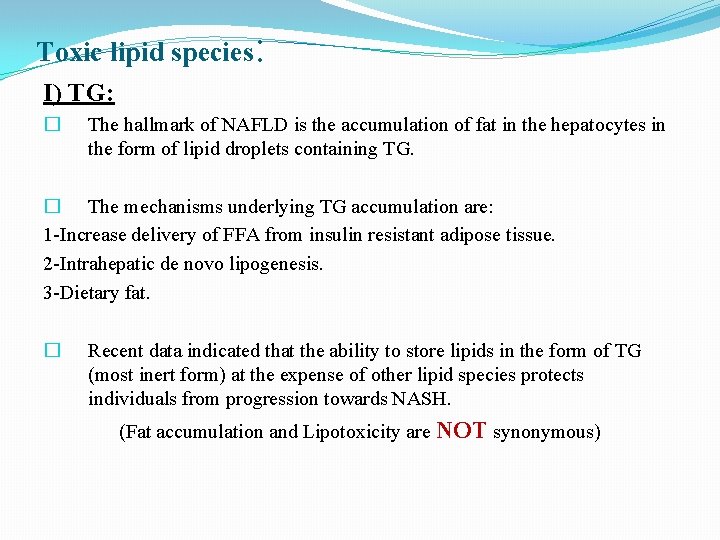
Toxic lipid species : I) TG: � The hallmark of NAFLD is the accumulation of fat in the hepatocytes in the form of lipid droplets containing TG. � The mechanisms underlying TG accumulation are: 1 -Increase delivery of FFA from insulin resistant adipose tissue. 2 -Intrahepatic de novo lipogenesis. 3 -Dietary fat. � Recent data indicated that the ability to store lipids in the form of TG (most inert form) at the expense of other lipid species protects individuals from progression towards NASH. (Fat accumulation and Lipotoxicity are NOT synonymous)

II) FFAs: Recent studies highlighted the potent toxic effects of unsaturated FFA particularly palmitate, oleate and stearate (they are major components of the diet or may be synthesized de novo from CHO). These FFAs induce cellular inflammation and apoptosis.

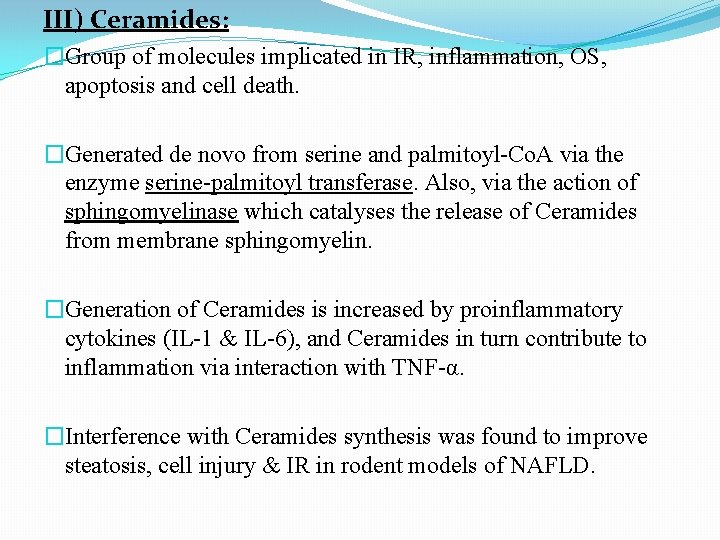
III) Ceramides: �Group of molecules implicated in IR, inflammation, OS, apoptosis and cell death. �Generated de novo from serine and palmitoyl-Co. A via the enzyme serine-palmitoyl transferase. Also, via the action of sphingomyelinase which catalyses the release of Ceramides from membrane sphingomyelin. �Generation of Ceramides is increased by proinflammatory cytokines (IL-1 & IL-6), and Ceramides in turn contribute to inflammation via interaction with TNF-α. �Interference with Ceramides synthesis was found to improve steatosis, cell injury & IR in rodent models of NAFLD.

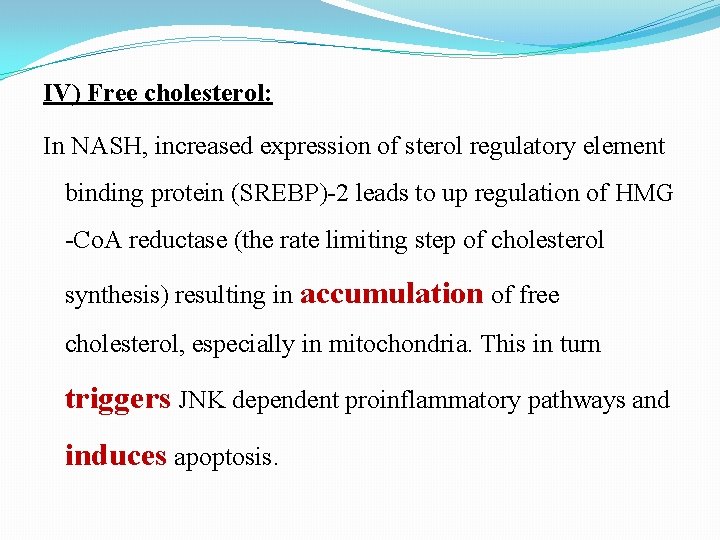
IV) Free cholesterol: In NASH, increased expression of sterol regulatory element binding protein (SREBP)-2 leads to up regulation of HMG -Co. A reductase (the rate limiting step of cholesterol synthesis) resulting in accumulation of free cholesterol, especially in mitochondria. This in turn triggers JNK dependent proinflammatory pathways and induces apoptosis.

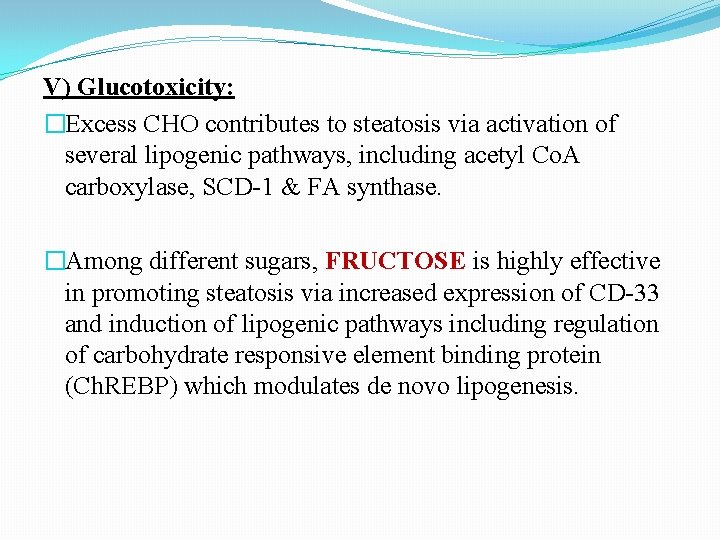
V) Glucotoxicity: �Excess CHO contributes to steatosis via activation of several lipogenic pathways, including acetyl Co. A carboxylase, SCD-1 & FA synthase. �Among different sugars, FRUCTOSE is highly effective in promoting steatosis via increased expression of CD-33 and induction of lipogenic pathways including regulation of carbohydrate responsive element binding protein (Ch. REBP) which modulates de novo lipogenesis.

Gut-Liver Axis: I) Gut microbiota and NAFLD. II) Gut hormones and the liver.

Gut microbiota and NAFLD: �Gut microbiota is defined as the microorganisms harboured by each person and is characterized by a high number of genes collectively called the microbiome, which is not equally distributed along the intestine, since the number of species increases from the esophagus to the rectum. �Normal human gut is colonized by a large number of microorganisms, at least 100 trillion, which contribute to various functions such as digestion, vitamin synthesis and resistance to colonization of the intestine by pathogens. �Qualitative and quantitative modification of gut microbiota called dysbiosis, which can be considered a predisposing factor for the development and progression of several chronic diseases related to metabolic alterations including NAFLD.

�Specific mechanisms are linking gut microbiota to obesity and liver NAFLD/NASH: *Proof: When gut microbiota from obese rodents were transferred to lean rodents, the recipients acquired the same metabolic alterations as the donors. Thus, recent ongoing researches and clinical trials study fecal microbiota transplantation as a therapeutic option in patients with NAFLD/NASH. *Theoretical mechanisms: 1 -Contribution to lipid accumulation in the liver by the endproducts of bacterial metabolism in the colon. Microbiota can ferment CHO to produce short chain fatty acids (SCFAs) which can directly act as lipid precursors in the liver. 2 -Altered microbiota (dysbiosis) inhibits the synthesis of fasting induced adipocyte factor (FIAF), a specific inhibitor of lipoprotein lipase resulting in higher accumulation of lipids in the liver.

3 -Translocation of bacteria or bacterial products into portal circulation, where bacterial translocation is followed by activation of pro-inflammatory pathways in the liver after binding to specific hepatic receptors (TLRs). TLRs possess different affinities for specific pathogens as follows: TLR 2: bind G +ve bacteria TLR 4: bind G –ve bacteria TLR 5: bind bacterial flagellin TLR 9: bind double stranded DNA bacteria Bacterial binding with different TLRs induces intracellular signaling cascade with secretion of active cytokines which are involved in inflammation & cell death. 4 -Patients with NASH showed increased abundance of ethanol producing bacteria in their gut microbiome and increased blood concentration of ethanol, compared to controls and patients with NAFLD. This indicates the possible role of alcohol producing microbiota in the pathogenesis of NASH.


Gut hormones and the liver: �Gut hormones (Incretins) are hormones released from the gut directly into the bloodstream in response to ingestion of food, they modulate the insulin secretory response where incretins account for at least 50% of the total insulin secreted. �The two best studied incretins are glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1), both incretins are rapidly deactivated by an enzyme called dipeptidyl peptidase 4 (DPP 4).


�Incretin-based therapies are among the most promising therapies for type 2 DM. Two strategies are available: 1 -Incretin mimetic (exenatide “Victoza”, SC injection, potent longacting agonist of the GLP-1 receptor). 2 -Incretin enhancer (DPP 4 inhibitors, Vildagliptin). �Recent studies on NAFLD/NASH patients revealed a significant reduction in serum ALT, steatosis, inflammation, and fibrosis following treatment with Incretin-based therapies (GLP-1 receptor agonists and DPP-4 inhibitors). However, further studies are needed to assess the long term effect of incretin based therapies on NAFLD/NASH.

*Incretin as a Novel Treatment Strategy for NAFLD/NASH. (2016) *Incretin-based therapies for the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. (J Gastroenterol Hepatol 2016) *Treatment options for alcoholic and non-alcoholic fatty liver disease: A review. (World J Gastroenterol 2017) *Current and future pharmacological therapies for NAFLD/NASH. (J Gastroenterol 2018) * The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver. (Hepatology 2018)

�EASL, EASD, EASO and recently AASLD proposed NAFLD/NASH “guidance” in which pioglitazone and vitamin E are recommended as pharmacotherapies for biopsy-proven NASH patients with fibrosis stage 2 or higher/with early stage fibrosis and high risk of fibrosis progression (older age, diabetes, metabolic syndrome, increased ALT, and high necroinflammatory activity), with and without diabetes. �In addition to pharmacotherapies, dietary changes; lifestyle modifications and body weight reduction are needed.

Conclusions: �Lipotoxicity & gut-liver axis (with its two components) are main contributors in the pathogenesis of NASH. �Fat accumulation and Lipotoxicity are NOT synonymous. �More researches and clinical trials are needed to confirm the role of fecal microbiota transplantation as a therapeutic option in patients with NAFLD/NASH. �More clinical studies are needed to confirm the role of incretin-based therapies in NAFLD/NASH patients. �Identify current therapies for NAFLD/NASH and their indications.

THANK YOU
 Pitch factor
Pitch factor A six pole ,60 hz synchoronous machine
A six pole ,60 hz synchoronous machine Axis 1 and axis 2 disorders
Axis 1 and axis 2 disorders Transverse axis and conjugate axis
Transverse axis and conjugate axis Personality disorder vs mental illness
Personality disorder vs mental illness Conic sections table
Conic sections table Function of liver in digestion
Function of liver in digestion Gut and tripe room
Gut and tripe room Entrepreneurial mind frame heart flame and gut game meaning
Entrepreneurial mind frame heart flame and gut game meaning Azure web role worker role example
Azure web role worker role example Mir geht es gut und wie geht es dir
Mir geht es gut und wie geht es dir Slidetodoc.com
Slidetodoc.com Ruh dich gut aus
Ruh dich gut aus Leo galland leaky gut
Leo galland leaky gut Triploblastic animals with a blind gut
Triploblastic animals with a blind gut Gut g?tesiegel
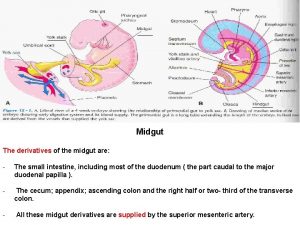
Gut g?tesiegel Midgut
Midgut Gut tube parts
Gut tube parts Guten tag hallo wie geht's
Guten tag hallo wie geht's Three disadvantages of having a blind gut in porifera
Three disadvantages of having a blind gut in porifera Besser schlecht gefahren als gut gelaufen
Besser schlecht gefahren als gut gelaufen Mind gut connection
Mind gut connection Midgut derivatives
Midgut derivatives