REPORT ON ACTIVITIES IN AFRICA FOR 2009 CITI







- Slides: 19

REPORT ON ACTIVITIES IN AFRICA FOR 2009 CITI SPRING MEETING Presented by rof. Ifeoma Okoye, President, Association for Good Clinical Practice in Nigeria (AGCPN) rofessor of Radiology, College of Medicine, University of Nigeria, Nsukka & University of Nigeria eaching Hospital (UNTH) Enugu, Nigeria FRICAN COORDINATOR OF CITI (Collaborative Institutional Training Initiative)

ASSOCIATION FOR GOOD CLINICAL PRACTICE IN NIGERIA (AGCPN) • an initiative that has been promoting responsible conduct of Human Research and developing the infrastructure for clinical trials in Nigeria through government-regulatory body advocacy /media sensitisation, conferences and training workshops. • an umbrella organisation networking investigators/clinical trialists in Nigeria

Association for Good Clinical Practices in Nigeria (AGCPN) (www. agcpn. org ) conducted Annual capacity building National training workshops in Nigeria for the past 4 years Over 250 GCP sensitized potential investigators

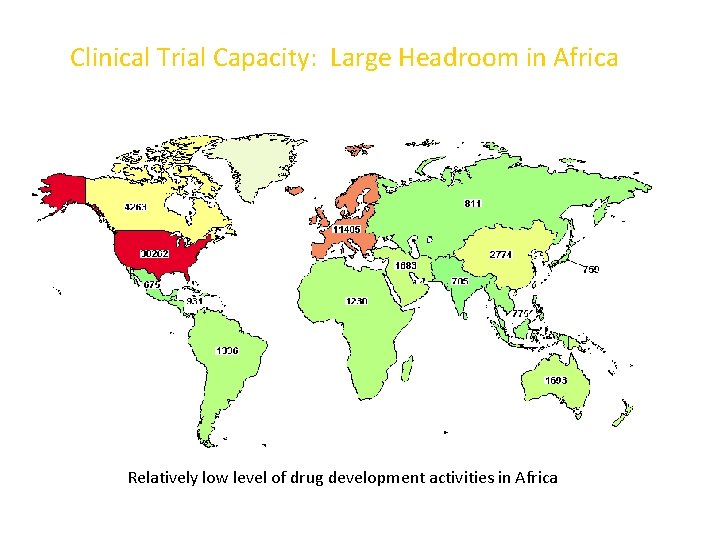
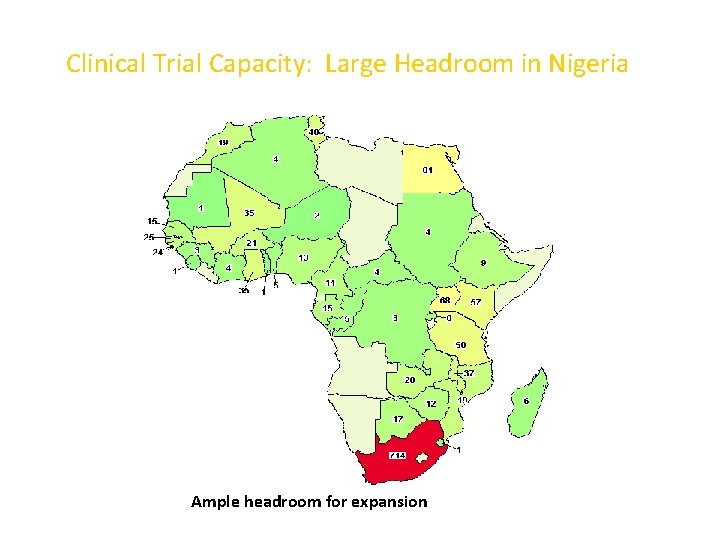
DEARTH OF INFLOW OF RCTS • 100, 000 clinical trials are carried out worldwide each year, of the total; only about 10% of them is performed in the developing countries, 1% in Africa and very minimal fraction performed in Nigeria • Available data show that out of the 1600 clinical trials conducted in Africa only about 1% is placed in Nigeria as compared to about 50% placed in South Africa, 6% in Egypt, 55% in Uganda and 3. 9% in Kenya. • Nigeria has not had significant HIV/AIDS trials like other countries in Eastern/Southern Africa

Clinical Trial Capacity: Large Headroom in Africa Relatively low level of drug development activities in Africa

Clinical Trial Capacity: Large Headroom in Nigeria Ample headroom for expansion

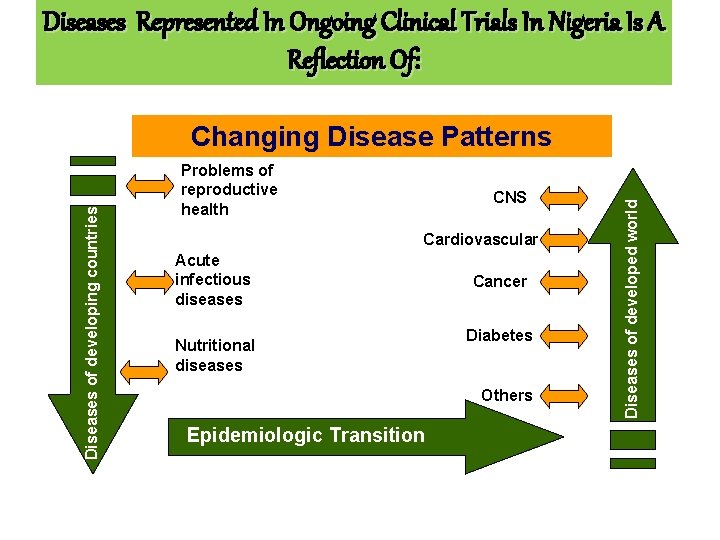
Diseases Represented In Ongoing Clinical Trials In Nigeria Is A Reflection Of: Problems of reproductive health CNS Cardiovascular Acute infectious diseases Nutritional diseases Cancer Diabetes Others Epidemiologic Transition Diseases of developed world Diseases of developing countries Changing Disease Patterns

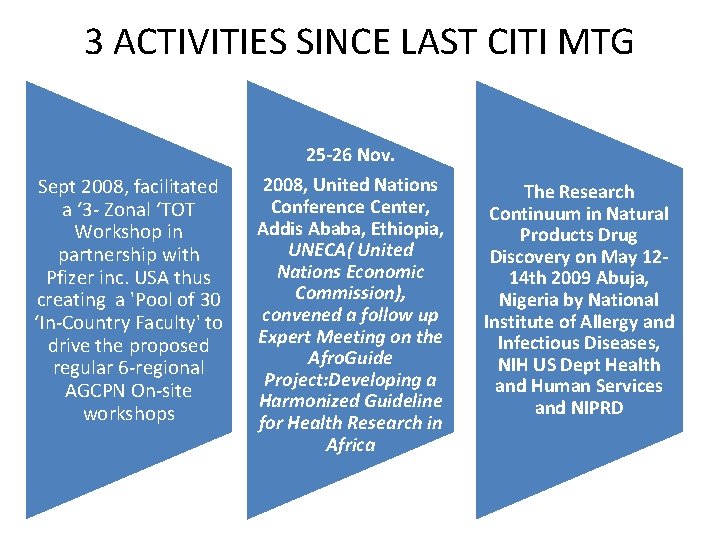
3 ACTIVITIES SINCE LAST CITI MTG 25 -26 Nov. Sept 2008, facilitated a ‘ 3 - Zonal ‘TOT Workshop in partnership with Pfizer inc. USA thus creating a 'Pool of 30 ‘In-Country Faculty' to drive the proposed regular 6 -regional AGCPN On-site workshops 2008, United Nations Conference Center, Addis Ababa, Ethiopia, UNECA( United Nations Economic Commission), convened a follow up Expert Meeting on the Afro. Guide Project: Developing a Harmonized Guideline for Health Research in Africa The Research Continuum in Natural Products Drug Discovery on May 1214 th 2009 Abuja, Nigeria by National Institute of Allergy and Infectious Diseases, NIH US Dept Health and Human Services and NIPRD

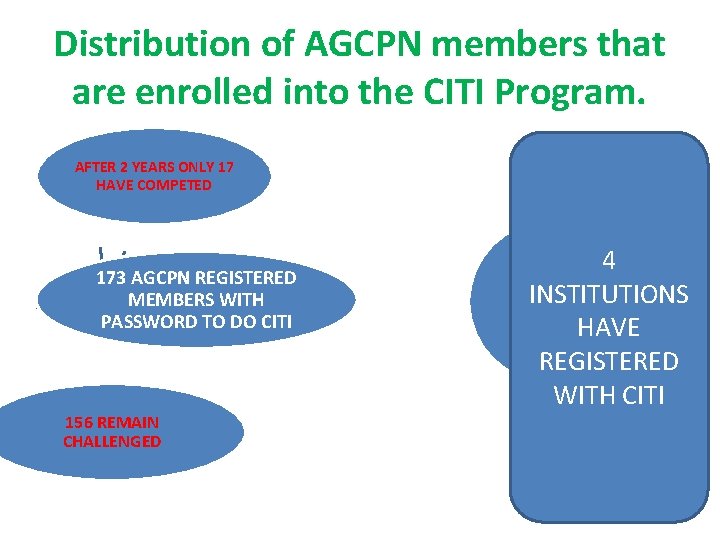
Distribution of AGCPN members that are enrolled into the CITI Program. AFTER 2 YEARS ONLY 17 HAVE COMPETED 173 AGCPN REGISTERED MEMBERS WITH PASSWORD TO DO CITI 156 REMAIN CHALLENGED 4 INSTITUTIONS HAVE REGISTERED WITH CITI

THE 4 REGISTERED INSTITUTIONS & the buy 4 get 1 free University of Nigeria Teaching Hospital, Ituku. Ozalla. (UNTH) Nnamdi Azikiwe University Teaching Hospital, Nnewi. (NAUTH). National Orthopaedic Hospital, Enugu. (NOHE). Ebonyi State University, Abakaliki. (EBSU) University of Nigeria Nsukka. (UNN)

Reasons advanced for this poor compliance Slow and scarcely available internet access… Makes the course laborious! (? IRB IN NOH) With dearth of research inflow into the country, no perceived benefits to training. No mandatory requirement of training by sponsors before becoming a PI THE 4 INSTITUTIONS HAVE NO CUSTOMIZED SITES DEVELOPED FOR THEM YET

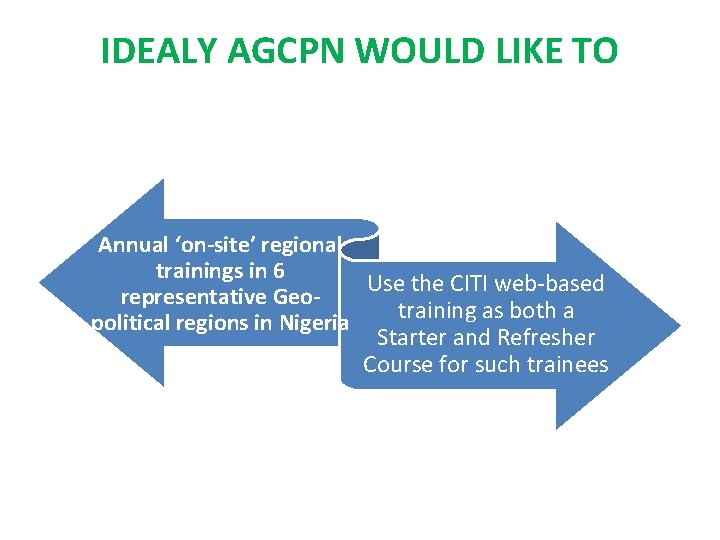
IDEALY AGCPN WOULD LIKE TO Annual ‘on-site’ regional trainings in 6 Use the CITI web-based representative Geotraining as both a political regions in Nigeria Starter and Refresher Course for such trainees

Participants at the opening ceremony of the Enugu Zonal To. T As AGCPN continues its advocacy to influence political will and commitment of policy makers to • Legislate and make mandatory RCR/GCP /Ethics/Bioethics Education for its research scientific community. • Invest in strengthening agencies like regulatory bodies, National/Institutional Ethics committees, and GCP initiatives through properly voted BUDGETRY ALLOCATION

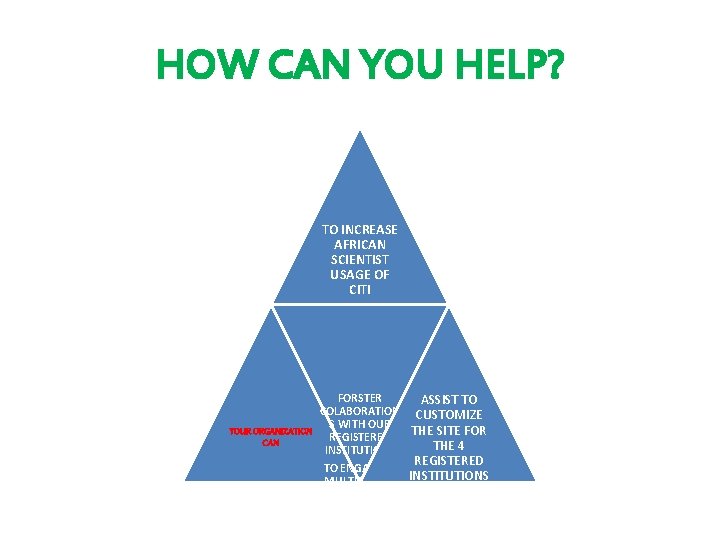
HOW CAN YOU HELP? TO INCREASE AFRICAN SCIENTIST USAGE OF CITI FORSTER ASSIST TO COLABORATION CUSTOMIZE S WITH OUR YOUR ORGANIZATION THE SITE FOR REGISTERED CAN THE 4 INSTITUTIONS REGISTERED TO ENGAGE IN INSTITUTIONS MULTICENTRE RESEARCH

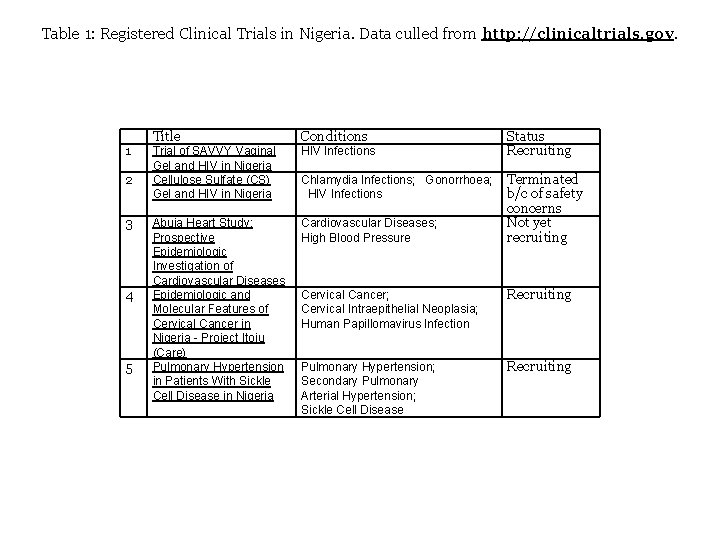
Table 1: Registered Clinical Trials in Nigeria. Data culled from http: //clinicaltrials. gov. 1 2 3 4 5 Title Conditions Trial of SAVVY Vaginal Gel and HIV in Nigeria Cellulose Sulfate (CS) Gel and HIV in Nigeria HIV Infections Abuja Heart Study: Prospective Epidemiologic Investigation of Cardiovascular Diseases Epidemiologic and Molecular Features of Cervical Cancer in Nigeria - Project Itoju (Care) Pulmonary Hypertension in Patients With Sickle Cell Disease in Nigeria Cardiovascular Diseases; High Blood Pressure Status Recruiting Chlamydia Infections; Gonorrhoea; Terminated b/c of safety HIV Infections concerns Not yet recruiting Cervical Cancer; Cervical Intraepithelial Neoplasia; Human Papillomavirus Infection Recruiting Pulmonary Hypertension; Secondary Pulmonary Arterial Hypertension; Sickle Cell Disease Recruiting

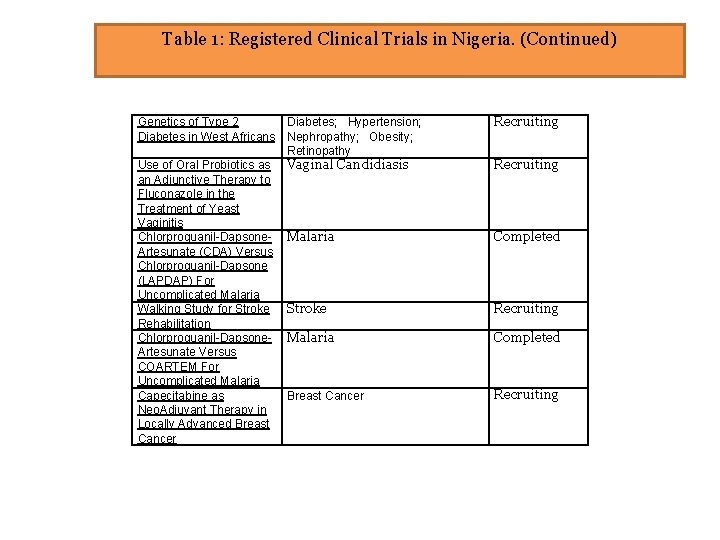
Table 1: Registered Clinical Trials in Nigeria. (Continued) Diabetes; Hypertension; Genetics of Type 2 Diabetes in West Africans Nephropathy; Obesity; Retinopathy Use of Oral Probiotics as Vaginal Candidiasis an Adjunctive Therapy to Fluconazole in the Treatment of Yeast Vaginitis Chlorproguanil-Dapsone- Malaria Artesunate (CDA) Versus Chlorproguanil-Dapsone (LAPDAP) For Uncomplicated Malaria Walking Study for Stroke Rehabilitation Chlorproguanil-Dapsone- Malaria Artesunate Versus COARTEM For Uncomplicated Malaria Capecitabine as Breast Cancer Neo. Adjuvant Therapy in Locally Advanced Breast Cancer Recruiting Completed Recruiting

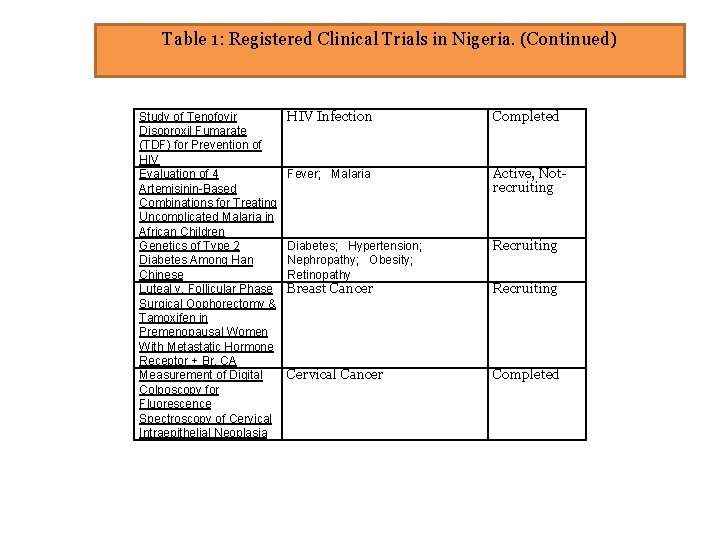
Table 1: Registered Clinical Trials in Nigeria. (Continued) Study of Tenofovir Disoproxil Fumarate (TDF) for Prevention of HIV Evaluation of 4 Artemisinin-Based Combinations for Treating Uncomplicated Malaria in African Children Genetics of Type 2 Diabetes Among Han Chinese Luteal v. Follicular Phase Surgical Oophorectomy & Tamoxifen in Premenopausal Women With Metastatic Hormone Receptor + Br. CA Measurement of Digital Colposcopy for Fluorescence Spectroscopy of Cervical Intraepithelial Neoplasia HIV Infection Completed Fever; Malaria Active, Notrecruiting Diabetes; Hypertension; Nephropathy; Obesity; Retinopathy Recruiting Breast Cancer Recruiting Cervical Cancer Completed

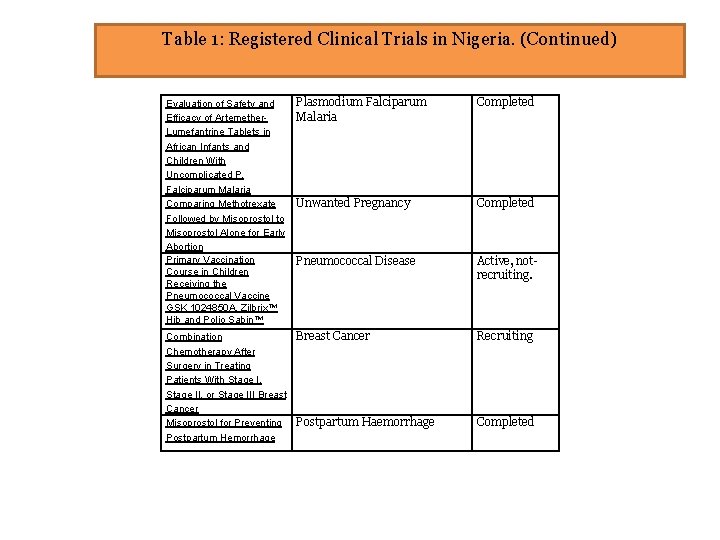
Table 1: Registered Clinical Trials in Nigeria. (Continued) Evaluation of Safety and Efficacy of Artemether. Lumefantrine Tablets in African Infants and Children With Uncomplicated P. Falciparum Malaria Comparing Methotrexate Followed by Misoprostol to Misoprostol Alone for Early Abortion Primary Vaccination Course in Children Receiving the Pneumococcal Vaccine GSK 1024850 A, Zilbrix™ Hib and Polio Sabin™ Plasmodium Falciparum Malaria Completed Unwanted Pregnancy Completed Pneumococcal Disease Active, notrecruiting. Combination Chemotherapy After Surgery in Treating Patients With Stage I, Stage II, or Stage III Breast Cancer Misoprostol for Preventing Postpartum Hemorrhage Breast Cancer Recruiting Postpartum Haemorrhage Completed

THE TASK MAY APPEAR HERCULIAN BUT IT IS DOABLE WITH ALL HANDS ON DECK!
 [email protected]
[email protected] Learning without burden is related to which committee
Learning without burden is related to which committee Aeis report 2009
Aeis report 2009 The statement of cash flows helps users
The statement of cash flows helps users Outdoor sports name
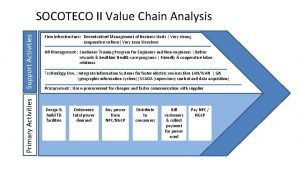
Outdoor sports name Primary and support activities
Primary and support activities Examples of primary activities
Examples of primary activities Nfsv
Nfsv Drexel citi training
Drexel citi training Uvm citi training
Uvm citi training Wvu citi training
Wvu citi training Citi commercial card
Citi commercial card Epikritické čití
Epikritické čití Citi mission statement
Citi mission statement Citi training ohsu
Citi training ohsu Drexel citi training
Drexel citi training Cassandra trigger example
Cassandra trigger example Citi training ub
Citi training ub Purdue irb
Purdue irb Carnegie mellon university research participants
Carnegie mellon university research participants