Regulatory Considerations for Approval FDA perspective Charu Mullick



![Trial Designs Design Add-on Superiority PBO control; Truvada in background [VRC 01 men, women] Trial Designs Design Add-on Superiority PBO control; Truvada in background [VRC 01 men, women]](https://slidetodoc.com/presentation_image_h/1339e3587f3ec00dd4263a934d79cad2/image-6.jpg)



- Slides: 14

Regulatory Considerations for Approval: FDA perspective Charu Mullick, MD Medical Officer, Division of Antiviral Products Center for Drug Evaluation and Research, FDA 9 th IAS Conference on HIV Science, Paris July 26, 2017

Assessing efficacy of investigational Pr. EP products • Regulatory framework • Adequacy of clinical trial data • Role of in vitro and in vivo data • Considerations for future clinical trial designs 2

Legal Effectiveness Requirement for “Substantial evidence…that the drug will have the effect it purports or is represented to have… under the conditions of use…as recommended or suggested in the proposed labeling” [FD&C Act sec 505(d)] FDA Guidance for Industry Providing Clinical Evidence of Effectiveness for Human Drug and Biological Products 3

Substantial evidence in the context of HIV Pr. EP • Animal models – Currently, no validated model • Ex vivo models – Promising • • Need for clinical data Pr. EP trials Phase 1 data to assess safety Sufficient HIV endpoints to discern protective effects in Phase 3 Safety database to ensure tolerability Other populations 4

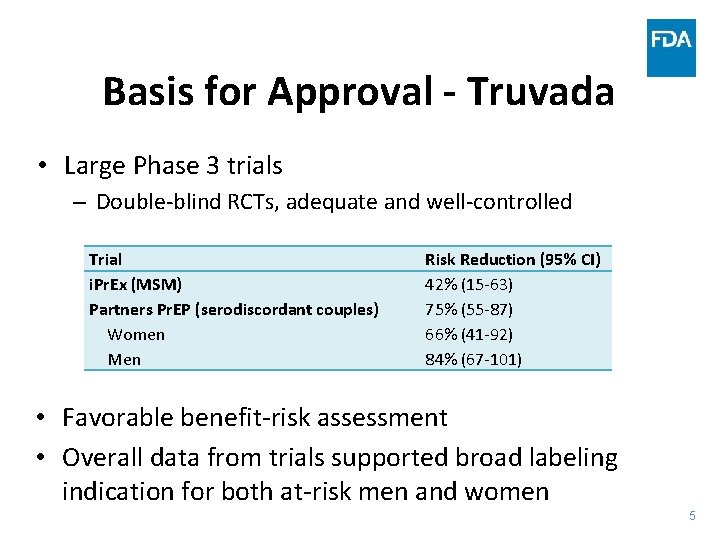
Basis for Approval - Truvada • Large Phase 3 trials – Double-blind RCTs, adequate and well-controlled Trial i. Pr. Ex (MSM) Partners Pr. EP (serodiscordant couples) Women Men Risk Reduction (95% CI) 42% (15 -63) 75% (55 -87) 66% (41 -92) 84% (67 -101) • Favorable benefit-risk assessment • Overall data from trials supported broad labeling indication for both at-risk men and women 5
![Trial Designs Design Addon Superiority PBO control Truvada in background VRC 01 men women Trial Designs Design Add-on Superiority PBO control; Truvada in background [VRC 01 men, women]](https://slidetodoc.com/presentation_image_h/1339e3587f3ec00dd4263a934d79cad2/image-6.jpg)
Trial Designs Design Add-on Superiority PBO control; Truvada in background [VRC 01 men, women] Active control Superiority [Cabotegravir women] Active control Noninferiority [F/TAF men] [Cabotegravir men] Single-arm trial with historical control Pros Concerns • Ethical issues if Truvada is not • Very large sample size approved or not accessible at trial sites Have the ability to • Long time to recruit, • obtain HIV endpoint data Large sample size needed - meet criteria for depends on level of adherence adequate and well Substantial delays in with Truvada controlled trial • Pr. EP development and NI trials in women - calculating an appropriate NI margin approval challenging Tempting…smaller sample size • Discouraged because of the uncertainty whether HIV incidence observed in the historical control is applicable to the current trial population 6

Oral Contraceptive Trials • Single arm trial • Use OC Pearl Index – Measure that is used to summarize contraceptive effectiveness – Based on historical evidence and confidence that vaginal sex leading to pregnancy will be occurring with a reliable frequency in a population/time period 7

Pr. EP trials • In MSM, rectal STIs are an indicator of unprotected receptive anal intercourse (RAI) • e. g. , rectal gonorrhea or chlamydia • Unprotected RAI is considered the major route of HIV acquisition Consideration for rectal STI incidence as a correlate or benchmark of HIV incidence in MSM 8

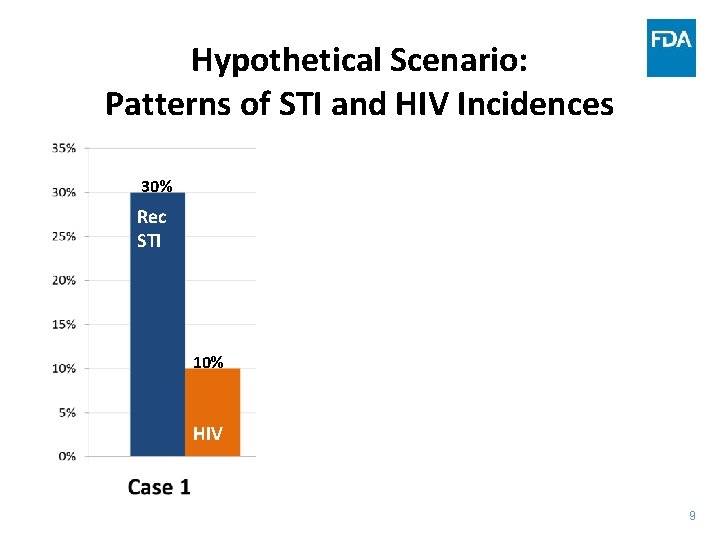
Hypothetical Scenario: Patterns of STI and HIV Incidences 30% Rec STI Expected HIV incidence with this rectal STI incidence: ~10% HIV 1% Case 2 9

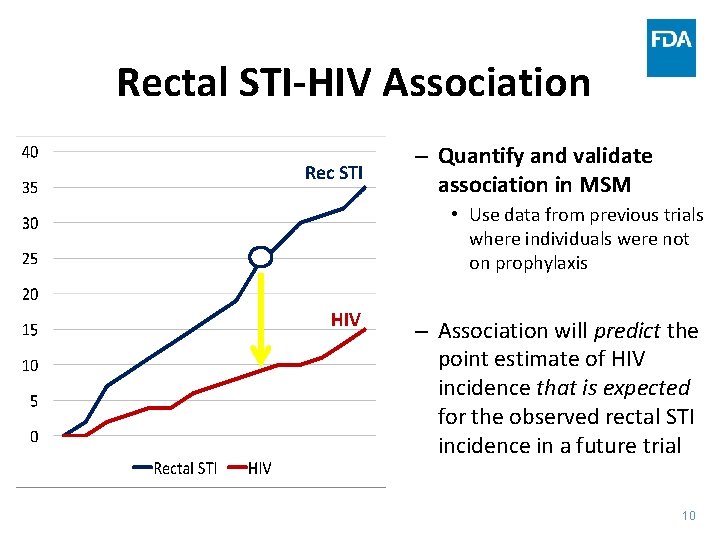
Rectal STI-HIV Association Rec STI – Quantify and validate association in MSM • Use data from previous trials where individuals were not on prophylaxis HIV – Association will predict the point estimate of HIV incidence that is expected for the observed rectal STI incidence in a future trial 10

Future Trial Design Implications • HIV incidence on investigational Pr. EP would be expected to be substantially lower than the lower bound of the quantitative estimate • Active controls could still be used for safety comparisons and an efficacy comparison (although underpowered) • Contingent on validating the rectal STI/HIV association 11

Limitations to Approach • Needs to be validated – Sufficient data to obtain a quantitative association – Many data points from multiple databases • Variable frequency of STI testing in older databases – May be less frequent than the current standard • Diagnostic assays for STIs in older databases – ? Less sensitive than currently used • Impact of successful Treatment as Prevention on recent HIV rates 12

Summary • We recognize the challenges with designing HIV Pr. EP trials • We are interested in working towards designing feasible and scientifically valid trials • Rectal gonorrhea/chlamydia incidence in MSM population can potentially serve as a benchmark that is predictive of HIV incidence – with trial design implications – Currently in exploration phase; the association is not yet validated • Similarly, benchmarks or HIV correlates identified in women could facilitate Pr. EP trial designs in women 13

Acknowledgements Jeff Murray Debra Birnkrant Kim Struble Pete Miele Thank you 14