Reactions In previous lectures materials flows were analyzed






- Slides: 11

Reactions In previous lectures materials flows were analyzed as steady-state processes. Time was not a variable. In many processes time variability is important.

Reaction Rates A mathematical expression describing the rate at which the mass or volume of some material A is changing with time T is: d. A/dt = r r = reaction rate Zero Order Reaction rate, r, is a constant: r=k So: d. A/dt = k

First Order Reactions The change of the component is proportional to the amount of the component present, or: r = k. A k is called the reaction rate constant the units on k are time-1 in the case of a first order reaction Second Order Reaction The change of the component is proportional to the square of the amount of the component present r = k A 2 So: d. A/dt = k A 2

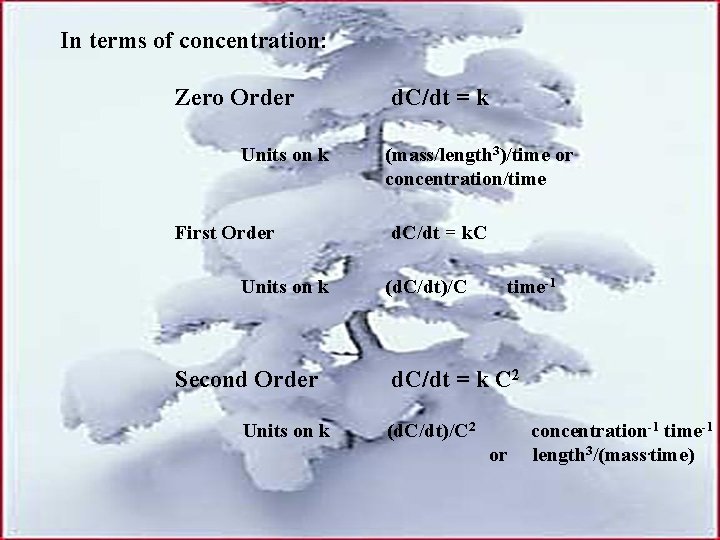
In terms of concentration: Zero Order Units on k First Order Units on k Second Order Units on k d. C/dt = k (mass/length 3)/time or concentration/time d. C/dt = k. C (d. C/dt)/C time-1 d. C/dt = k C 2 (d. C/dt)/C 2 or concentration-1 time-1 length 3/(mass. time)

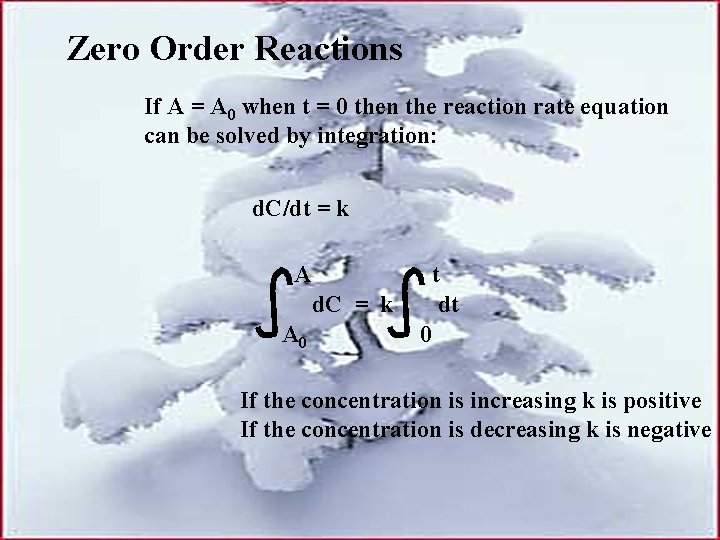
Zero Order Reactions If A = A 0 when t = 0 then the reaction rate equation can be solved by integration: d. C/dt = k A d. C = k A 0 t dt 0 If the concentration is increasing k is positive If the concentration is decreasing k is negative

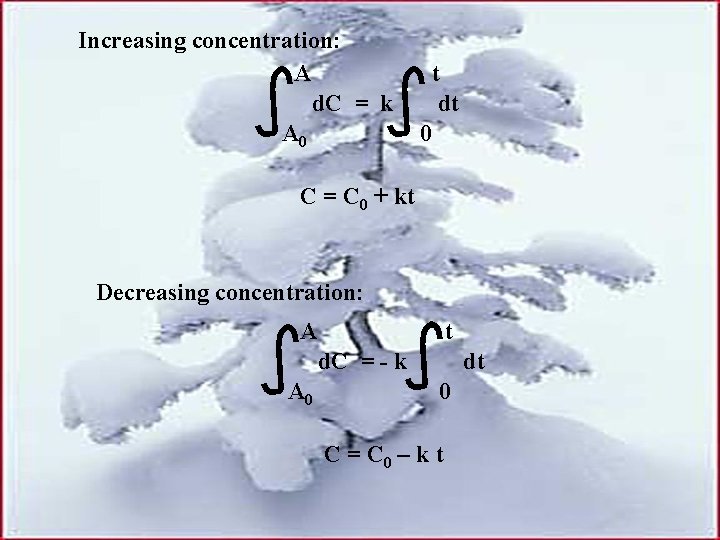
Increasing concentration: A d. C = k A 0 t dt 0 C = C 0 + kt Decreasing concentration: A t d. C = - k A 0 dt 0 C = C 0 – k t

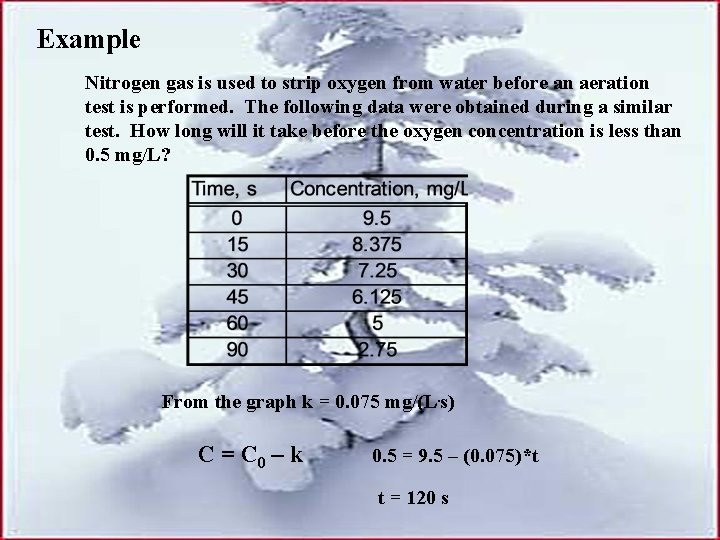
Example Nitrogen gas is used to strip oxygen from water before an aeration test is performed. The following data were obtained during a similar test. How long will it take before the oxygen concentration is less than 0. 5 mg/L? From the graph k = 0. 075 mg/(L. s) C = C 0 – k 0. 5 = 9. 5 – (0. 075)*t t = 120 s

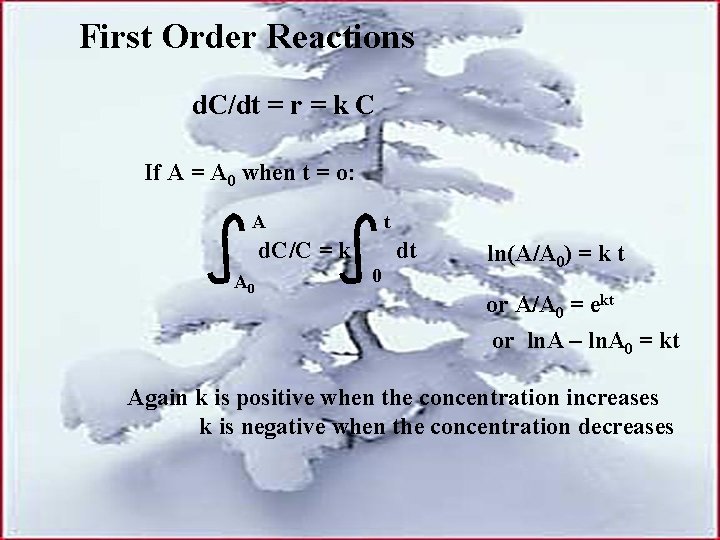
First Order Reactions d. C/dt = r = k C If A = A 0 when t = o: A t d. C/C = k A 0 dt 0 ln(A/A 0) = k t or A/A 0 = ekt or ln. A – ln. A 0 = kt Again k is positive when the concentration increases k is negative when the concentration decreases

ln A – ln A 0 = k t ln A = k t + ln A 0 y = mx+ b So, if we plot (ln A) on the y-axis and (t) on the x-axis The slope is the reaction rate constant, k, and the intercept is ln A 0

Example When the BOD of a wastewater is caused by particulate material its removal may often be described as first order. Consider the following data which were obtained from a batch test of a food processing waste water: How long will it take to reduce the BOD to 30 mg/L? What will the BOD be after 5 days?

So, k = 0. 011 hr-1 Now: ln(A/A 0) = -kt t = [ln(A/A 0)]/-k t = [ln(30/220)]/(-0. 011) = 181 hr = 7. 55 days Also: [BOD] = [BOD 0] e-kt = (220) e-(0. 011)(5 x 24) = 58. 8 mg/L