Radiometric Dating Timothy G Standish Ph D 1998





















- Slides: 41

Radiometric Dating Timothy G. Standish, Ph. D. © 1998 Timothy G. Standish

Dating Fossils Two methods: Relative dating - When a previously unknown fossil is found in strata with other fossils of “known age, ” the age of the newly discovered fossil can be inferred from the “known age” of the fossils it is associated with. Relative dating is done in terms of the relative appearance of organisms in the fossil record. (“Archaeopteryx appears after Latimeria, but before Australopithecus. ”) Absolute dating - Involves assigning dates in terms of years to fossils. This most frequently involves radiometric dating techniques. (“This Archaeopteryx fossil is 150 million years old. ”) © 1998 Timothy G. Standish

Radiometric Dating Assumptions: 1 Constant isotope decay rates over time 2 Initial isotope concentrations can be known 3 Isotope decay is the only factor that alters relative concentrations of isotopes and their breakdown products Ensuring that each of these assumptions is met can be very difficult if not impossible © 1998 Timothy G. Standish

Radio Isotope Dating To be the same, elements must have the same number of protons Isotopes are elements with the same number of protons, but different numbers of neutrons e. g. , uranium 235 (235 U) and 238 U each have 92 protons, but 143 and 146 neutrons respectively Some isotopes are more stable than others Unstable isotopes tend to decay over time to more stable forms In this decay process, a proton may be gained or lost changing the element © 1998 Timothy G. Standish

Radioisotope Dating If you can know the amount of an unstable isotope that was in a sample And you know the rate at which that isotope decays And the rate of decay has not changed over time And you can measure the amount of that isotope presently in the sample You can figure out how old the sample is © 1998 Timothy G. Standish

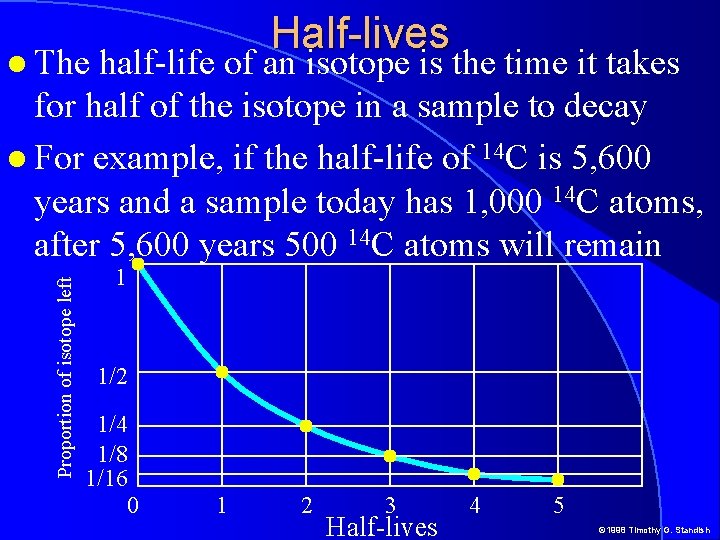
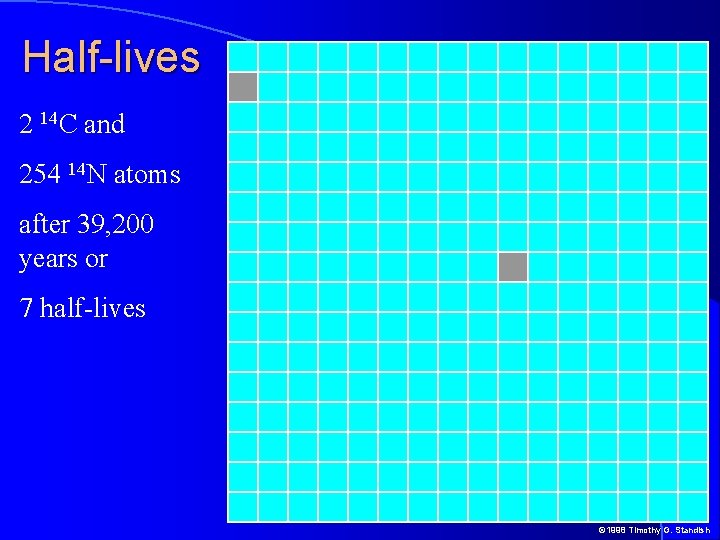
Half-lives half-life of an isotope is the time it takes for half of the isotope in a sample to decay For example, if the half-life of 14 C is 5, 600 years and a sample today has 1, 000 14 C atoms, after 5, 600 years 500 14 C atoms will remain Proportion of isotope left The 1 1/2 1/4 1/8 1/16 0 1 2 3 Half-lives 4 5 © 1998 Timothy G. Standish

Carbon-14 (14 C) a rare isotope of carbon, that has 6 protons and 8 neutrons 14 C decays to 14 N at a constant rate Every 5, 600 years half the 14 C in a sample will emit a beta particle (electron) and decay to 14 N Thus 5, 600 years is called the half-life of 14 C Because of 14 C’s short half-life, it is not useful for dating million year old fossils, it is only accurate to about 50, 000 years © 1998 Timothy G. Standish

Half-lives 256 14 C atoms at time 0 © 1998 Timothy G. Standish

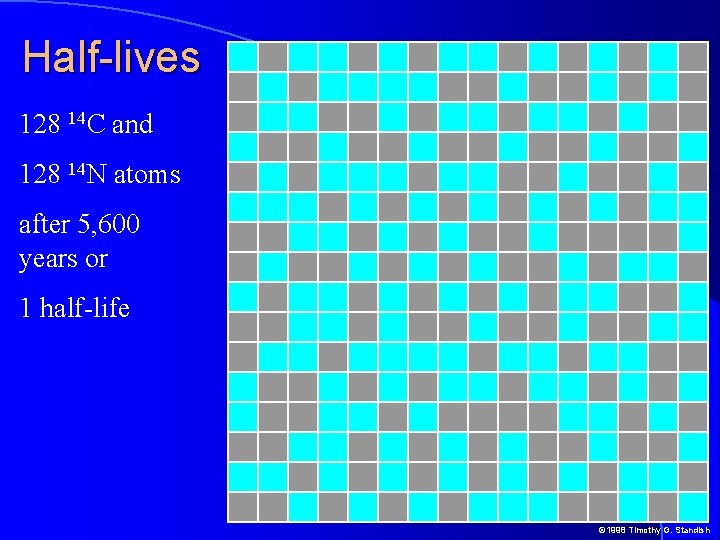
Half-lives 128 14 C and 128 14 N atoms after 5, 600 years or 1 half-life © 1998 Timothy G. Standish

Half-lives 64 14 C and 192 14 N atoms after 11, 200 years or 2 half-lives © 1998 Timothy G. Standish

Half-lives 32 14 C and 224 14 N atoms after 16, 800 years or 3 half-lives © 1998 Timothy G. Standish

Half-lives 16 14 C and 240 14 N atoms after 22, 400 years or 4 half-lives © 1998 Timothy G. Standish

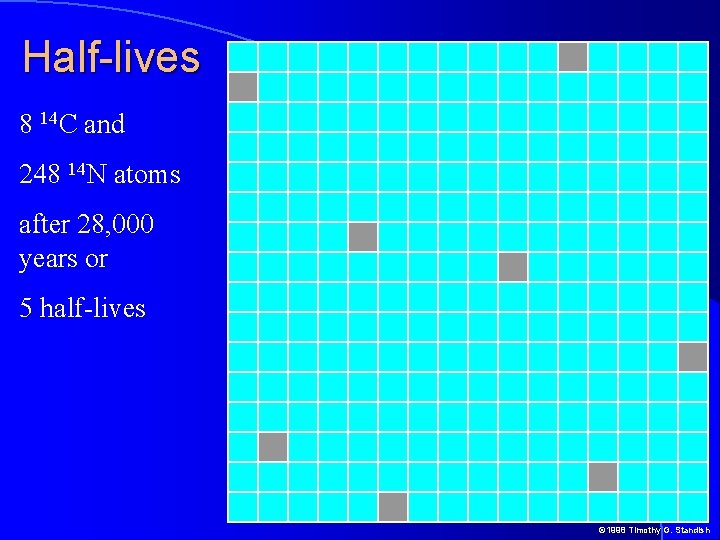
Half-lives 8 14 C and 248 14 N atoms after 28, 000 years or 5 half-lives © 1998 Timothy G. Standish

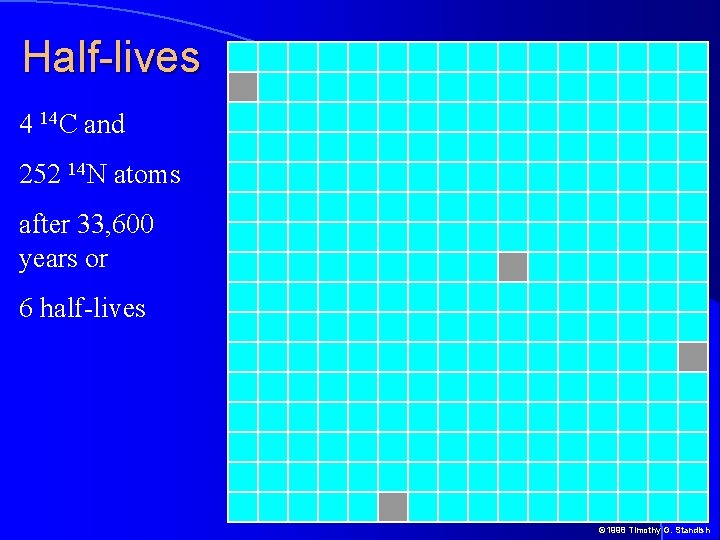
Half-lives 4 14 C and 252 14 N atoms after 33, 600 years or 6 half-lives © 1998 Timothy G. Standish

Half-lives 2 14 C and 254 14 N atoms after 39, 200 years or 7 half-lives © 1998 Timothy G. Standish

is used to date organic samples like wood, hair, shells (Ca. CO 3) and other plant and animal products Atmospheric 14 C is incorporated into organic molecules by plants during photosynthesis Animals that eat the plants get 14 C from the plants they eat The current ratio of 14 C to 12 C in the atmosphere is immeasurably small 14 C Carbon-14 © 1998 Timothy G. Standish

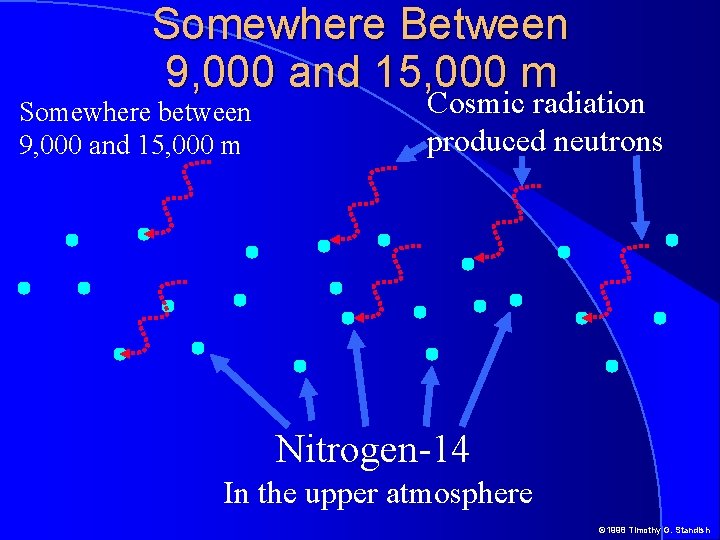
Carbon-14 With a relatively short half-life and an earth billions of years old, all C 14 should be gone This would be true if not for production of new 14 C in the atmosphere as a result of interactions between the upper atmosphere and neutrons in cosmic radiation The atmospheric ratio of 14 C to 12 C represents an equilibrium between production and decay of 14 C © 1998 Timothy G. Standish

Somewhere Between 9, 000 and 15, 000 m Somewhere between 9, 000 and 15, 000 m Cosmic radiation produced neutrons Nitrogen-14 In the upper atmosphere © 1998 Timothy G. Standish

Somewhere Between 9, 000 and 15, 000 m Carbon-14 In the upper atmosphere © 1998 Timothy G. Standish

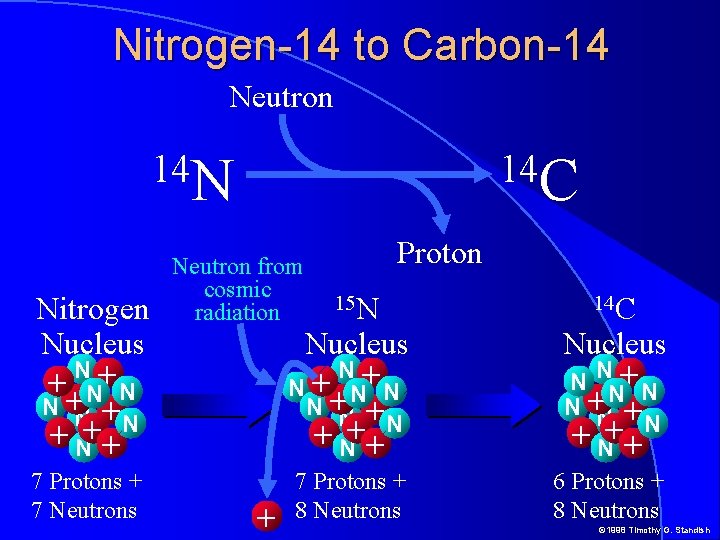
Nitrogen-14 to Carbon-14 Neutron 14 N Nitrogen Nucleus N+ ++N N + N + +N + 7 Protons + 7 Neutrons 14 C Neutron from cosmic radiation Proton 15 N Nucleus + N+ N+ N N N+ N +N + N++ 7 Protons + 8 Neutrons 14 C Nucleus N NN+N N+ N +N + N++ 6 Protons + 8 Neutrons © 1998 Timothy G. Standish

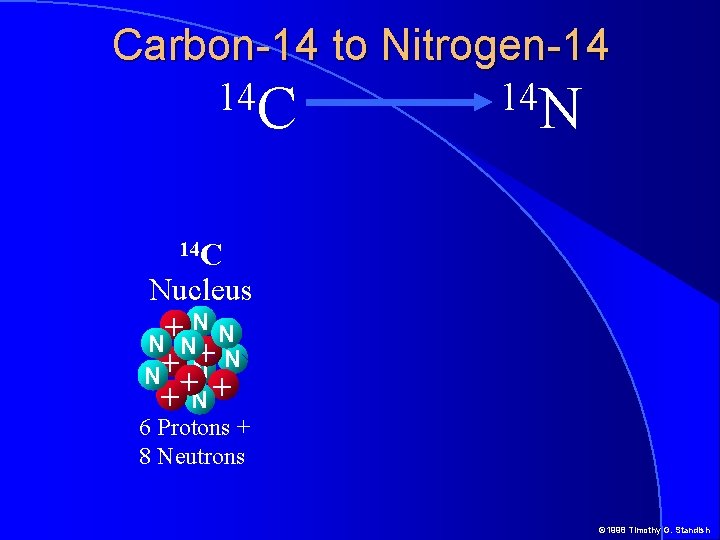
Carbon-14 to Nitrogen-14 14 C 14 N 14 C Nucleus NN + N N + + N+ 6 Protons + 8 Neutrons © 1998 Timothy G. Standish

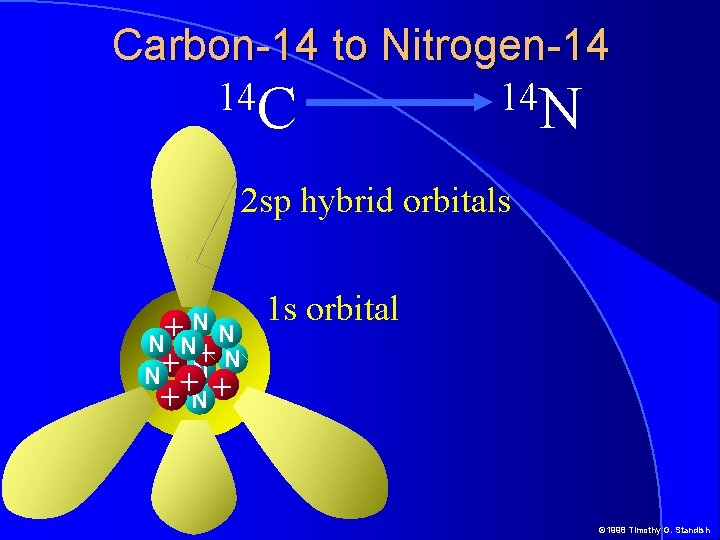
Carbon-14 to Nitrogen-14 14 C 14 N 2 sp hybrid orbitals NN + N N + + N+ 1 s orbital © 1998 Timothy G. Standish

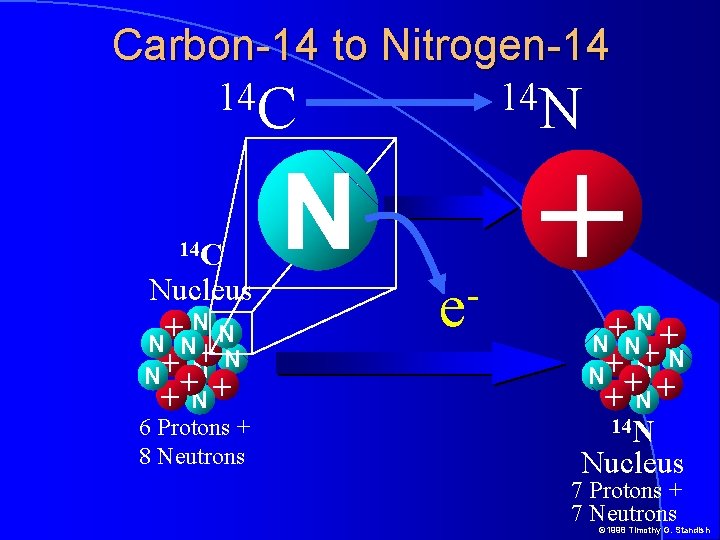
Carbon-14 to Nitrogen-14 14 C Nucleus NN + N N + + N+ 6 Protons + 8 Neutrons 14 N N e + N N + +N + N N + + N+ 14 N Nucleus 7 Protons + 7 Neutrons © 1998 Timothy G. Standish

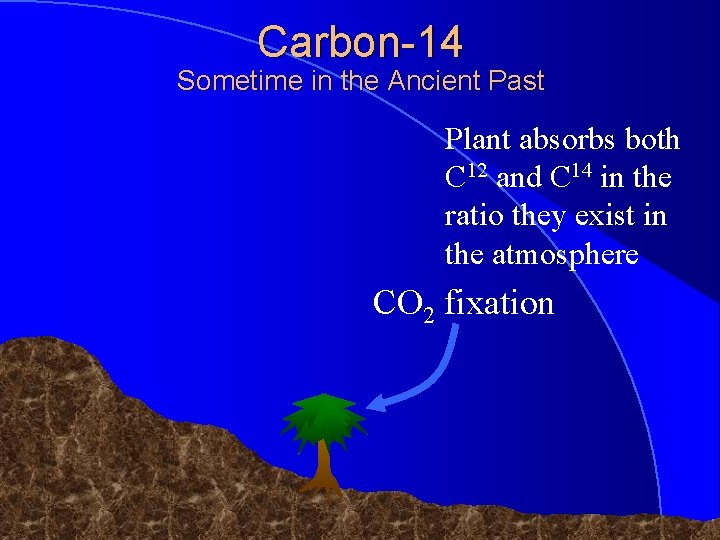
Carbon-14 Sometime in the Ancient Past Plant absorbs both C 12 and C 14 in the ratio they exist in the atmosphere CO 2 fixation © 1998 Timothy G. Standish

Carbon-14 A Plant Grows Absorbing CO 2 © 1998 Timothy G. Standish

Carbon-14 The Plant Dies © 1998 Timothy G. Standish

Carbon-14 It Is Buried © 1998 Timothy G. Standish

Carbon-14 Over Time 14 C Decays to 14 N © 1998 Timothy G. Standish

Carbon-14 Over Time 14 C Decays to 14 N © 1998 Timothy G. Standish

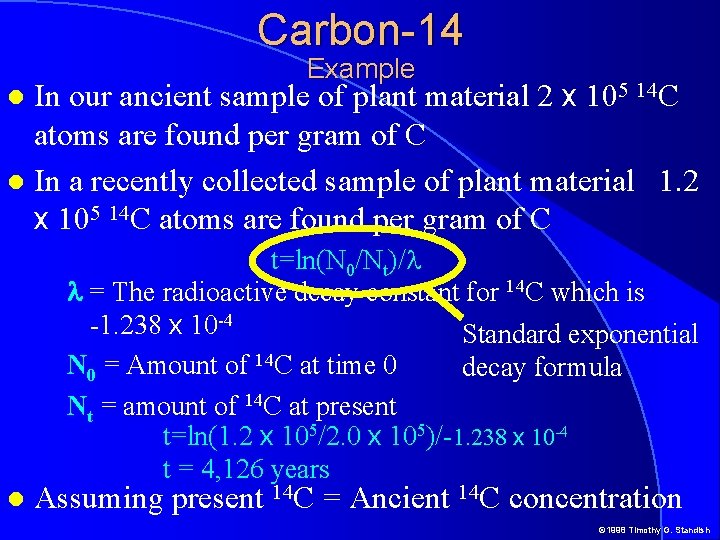
Carbon-14 Example In our ancient sample of plant material 2 x 105 14 C atoms are found per gram of C In a recently collected sample of plant material 1. 2 x 105 14 C atoms are found per gram of C t=ln(N 0/Nt)/l l = The radioactive decay constant for 14 C which is -1. 238 x 10 -4 Standard exponential N 0 = Amount of 14 C at time 0 decay formula Nt = amount of 14 C at present t=ln(1. 2 x 105/2. 0 x 105)/-1. 238 x 10 -4 t = 4, 126 years Assuming present 14 C = Ancient 14 C concentration © 1998 Timothy G. Standish

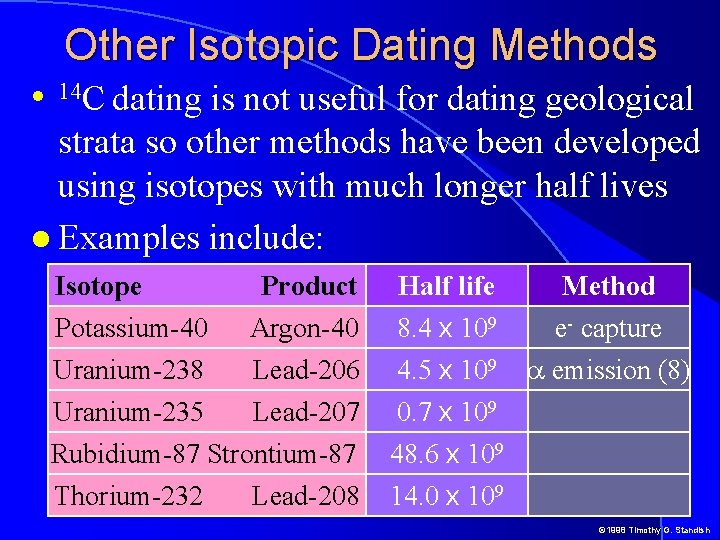
Other Isotopic Dating Methods is not useful for dating geological strata so other methods have been developed using isotopes with much longer half lives Examples include: 14 C dating Isotope Potassium-40 Uranium-238 Product Argon-40 Lead-206 Half life 8. 4 x 109 4. 5 x 109 Uranium-235 Lead-207 0. 7 x 109 Rubidium-87 Strontium-87 Thorium-232 Lead-208 48. 6 x 109 14. 0 x 109 Method e- capture a emission (8) © 1998 Timothy G. Standish

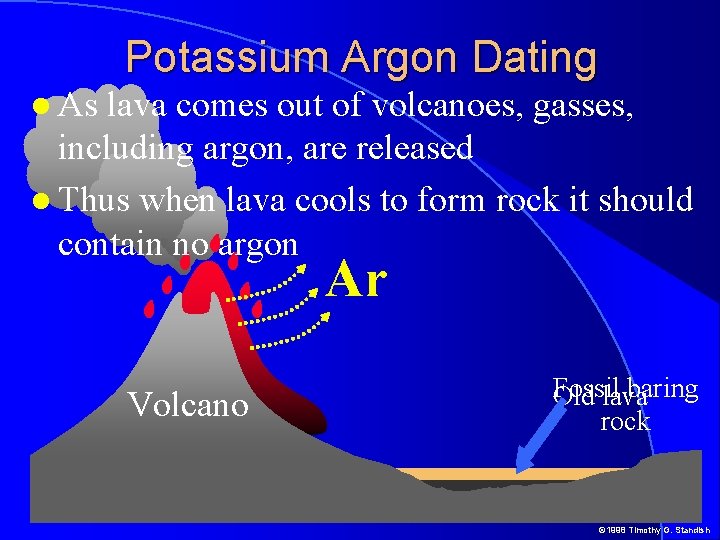
Potassium Argon Dating Potassium is abundant in rocks 40 K decays to 40 Ar and 40 Ca in a specific ratio, 11. 2 40 Ar to 88. 8 40 Ca As calcium is abundant in rocks, 40 Ca is not an easy isotope to use in dating In theory, all 40 Ar should be released as argon gas when igneous rock is formed Thus, during creation of new igneous rock, the potassium argon clock is set to zero. . . at least in theory © 1998 Timothy G. Standish

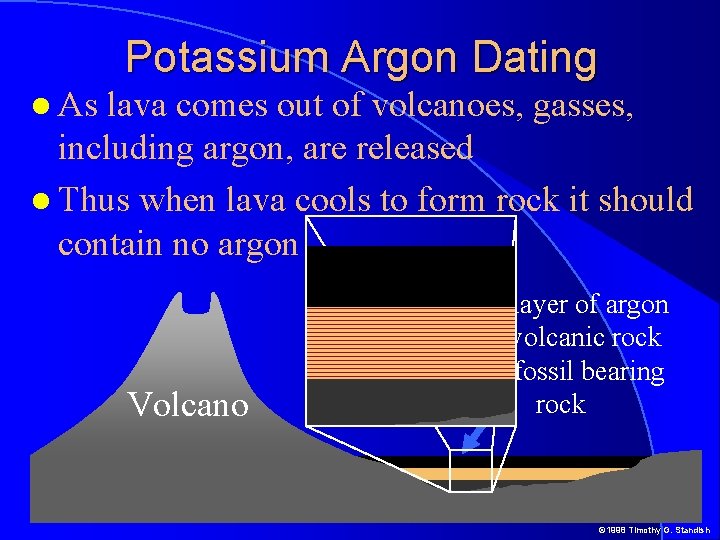
Potassium Argon Dating As lava comes out of volcanoes, gasses, including argon, are released Thus when lava cools to form rock it should contain no argon Ar Volcano Fossil baring Old lava rock © 1998 Timothy G. Standish

Potassium Argon Dating As lava comes out of volcanoes, gasses, including argon, are released Thus when lava cools to form rock it should contain no argon Volcano © 1998 Timothy G. Standish

Potassium Argon Dating As lava comes out of volcanoes, gasses, including argon, are released Thus when lava cools to form rock it should contain no argon Volcano New layer of argon free volcanic rock over fossil bearing rock © 1998 Timothy G. Standish

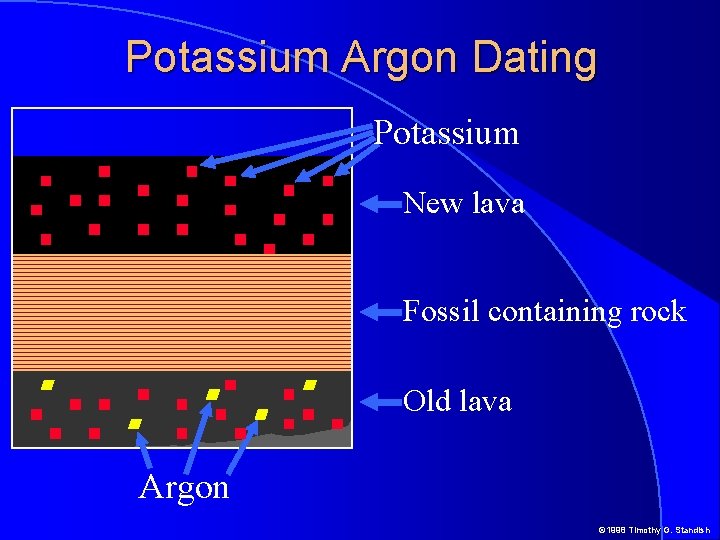
Potassium Argon Dating Potassium New lava Fossil containing rock Old lava Argon © 1998 Timothy G. Standish

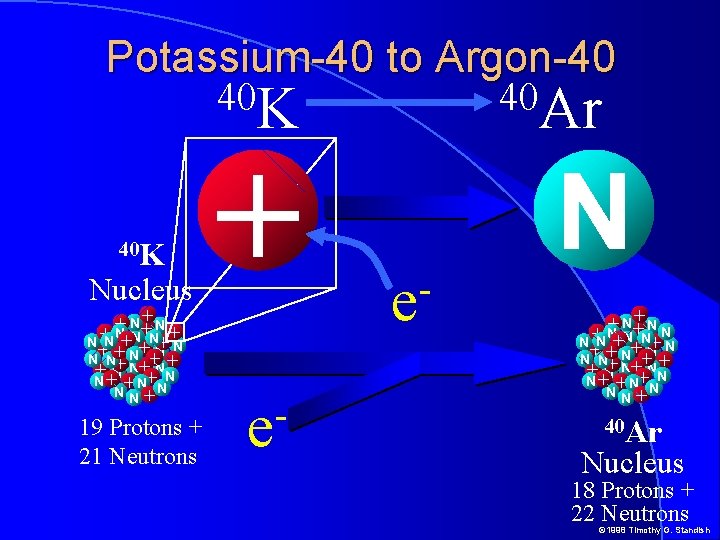
Potassium-40 to Argon-40 40 K Nucleus +N N+ + + +N N N +N N+ N + N++N N N+ + N+ N++NN+ N+ +N N NN+ 19 Protons + 21 Neutrons + e 40 Ar e N +N N+ + N +N N+ N + N++N N N+ + N+ N++NN+ N+ +N N NN+ 40 Ar Nucleus 18 Protons + 22 Neutrons © 1998 Timothy G. Standish

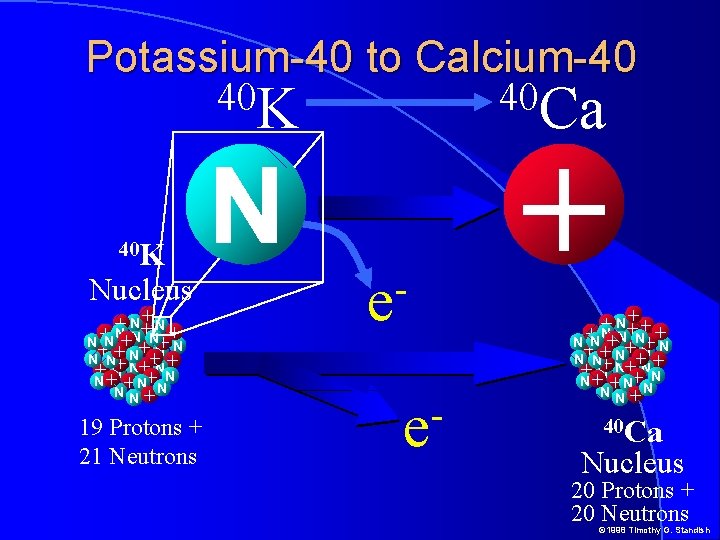
Potassium-40 to Calcium-40 40 K Nucleus +N N+ + + +N N N +N N+ N + N++N N N+ + N+ N++NN+ N+ +N N NN+ 19 Protons + 21 Neutrons 40 Ca N e e + + N+ + +N ++ N N +N N+ N + N++N N N+ + N+ N++NN+ N+ +N N NN+ 40 Ca Nucleus 20 Protons + 20 Neutrons © 1998 Timothy G. Standish

Potassium-Argon Dating Many years later Fossils found in strata above the old lava must be younger than it is Fossils in strata under the new lava must be older than it is Thus potassium-argon dating can give ages between which fossils must have formed Older Oldest Potassium Argon © 1998 Timothy G. Standish

When the Data Speaks “For example, researchers have calculated that 'mitochondrial Eve'--the woman whose mt. DNA was ancestral to that in all living people--lived 100, 000 to 200, 000 years ago in Africa. Using the new clock, she would be a mere 6, 000 years old. No one thinks that's the case, but at what point should models switch from one mt. DNA time zone to the other? ” Gibbons, A. 1998. Calibrating the mitochondrial clock. Science 279: 28 -29 © 1998 Timothy G. Standish

© 1998 Timothy G. Standish