Radicals and Radical Reactions Pensum Solomon Fryhle Chapter



- Slides: 22

Radicals and Radical Reactions Pensum: Solomon& Fryhle - Chapter 10, parts 10. 1 – 10. 4, 10. 11 A, B, E - Chapter 15, part 15. 12 A - Handout Chapter 10

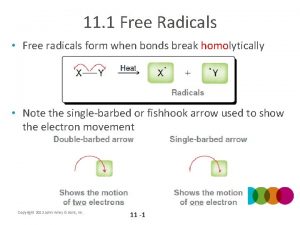
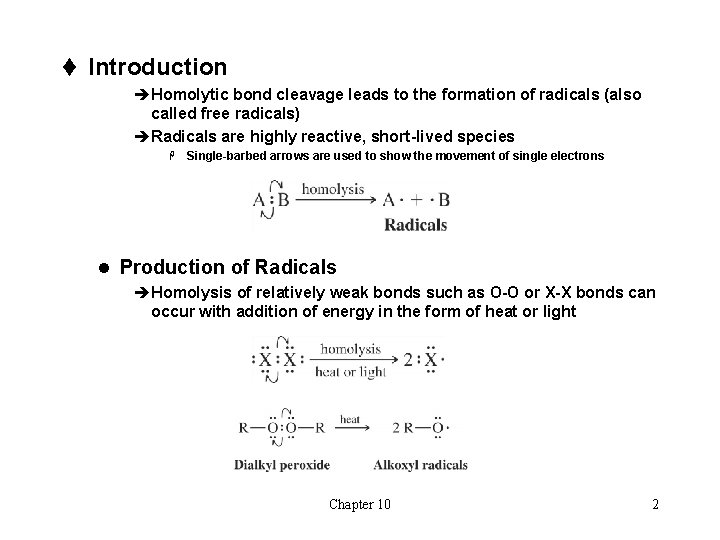
t Introduction èHomolytic bond cleavage leads to the formation of radicals (also called free radicals) èRadicals are highly reactive, short-lived species H Single-barbed arrows are used to show the movement of single electrons l Production of Radicals èHomolysis of relatively weak bonds such as O-O or X-X bonds can occur with addition of energy in the form of heat or light Chapter 10 2

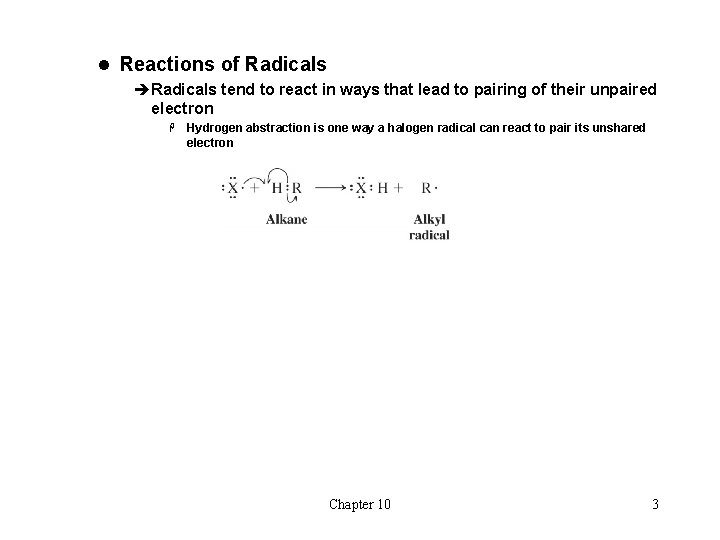
l Reactions of Radicals èRadicals tend to react in ways that lead to pairing of their unpaired electron H Hydrogen abstraction is one way a halogen radical can react to pair its unshared electron Chapter 10 3

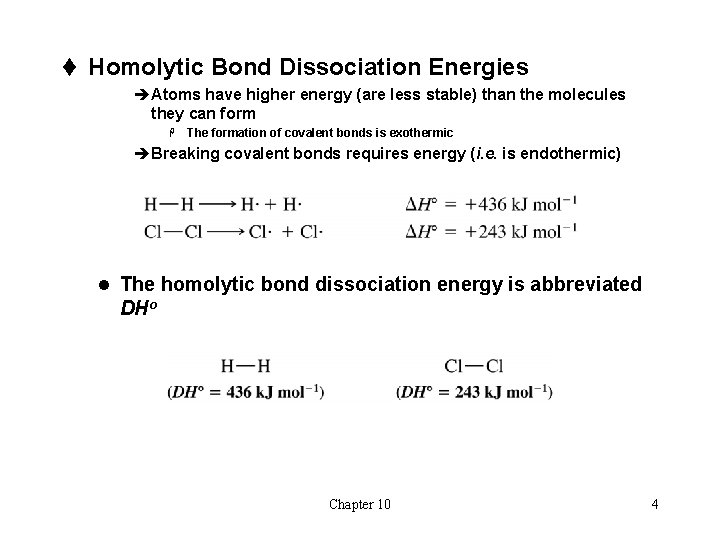
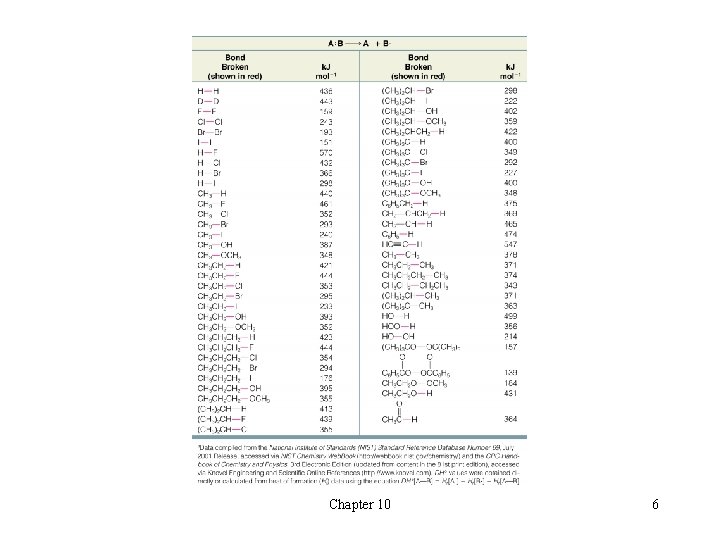
t Homolytic Bond Dissociation Energies èAtoms have higher energy (are less stable) than the molecules they can form H The formation of covalent bonds is exothermic èBreaking covalent bonds requires energy (i. e. is endothermic) l The homolytic bond dissociation energy is abbreviated DHo Chapter 10 4

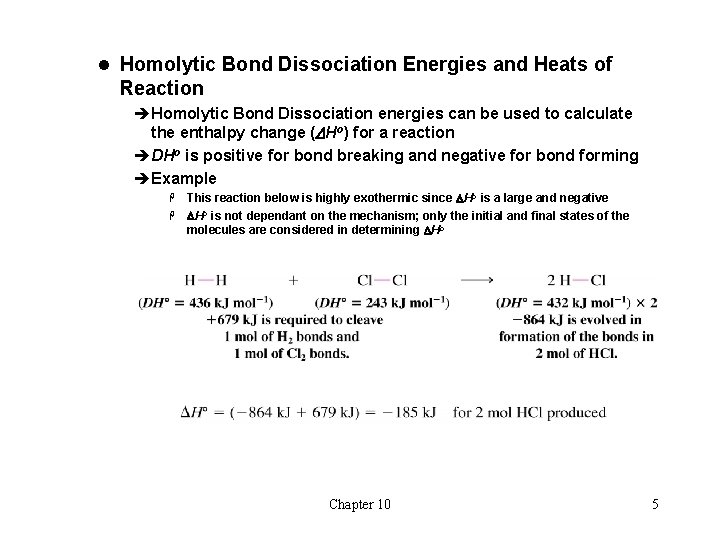
l Homolytic Bond Dissociation Energies and Heats of Reaction èHomolytic Bond Dissociation energies can be used to calculate the enthalpy change (DHo) for a reaction èDHo is positive for bond breaking and negative for bond forming èExample This reaction below is highly exothermic since DHo is a large and negative H DHo is not dependant on the mechanism; only the initial and final states of the molecules are considered in determining DHo H Chapter 10 5

Chapter 10 6

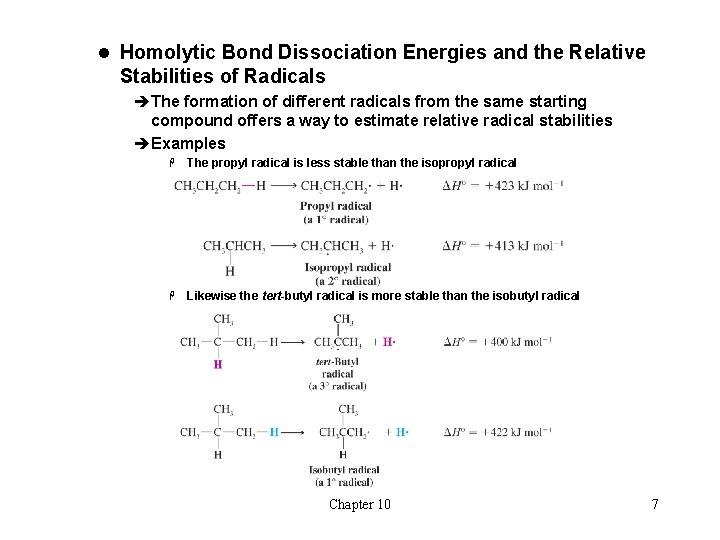
l Homolytic Bond Dissociation Energies and the Relative Stabilities of Radicals èThe formation of different radicals from the same starting compound offers a way to estimate relative radical stabilities èExamples H The propyl radical is less stable than the isopropyl radical H Likewise the tert-butyl radical is more stable than the isobutyl radical Chapter 10 7

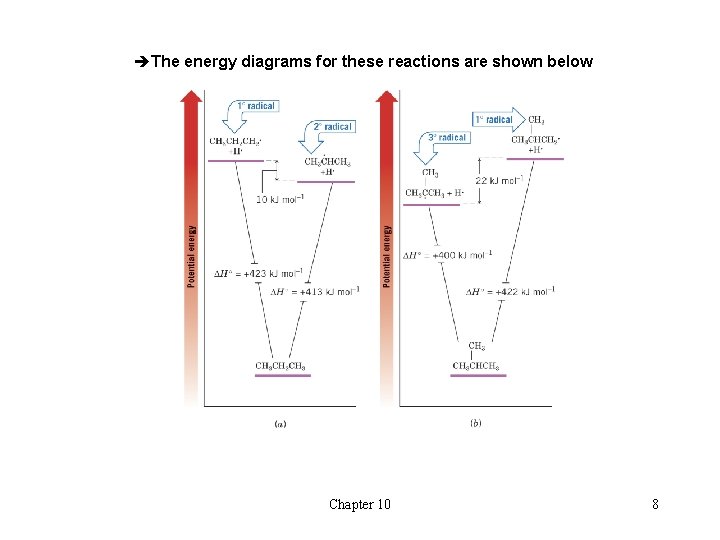
èThe energy diagrams for these reactions are shown below Chapter 10 8

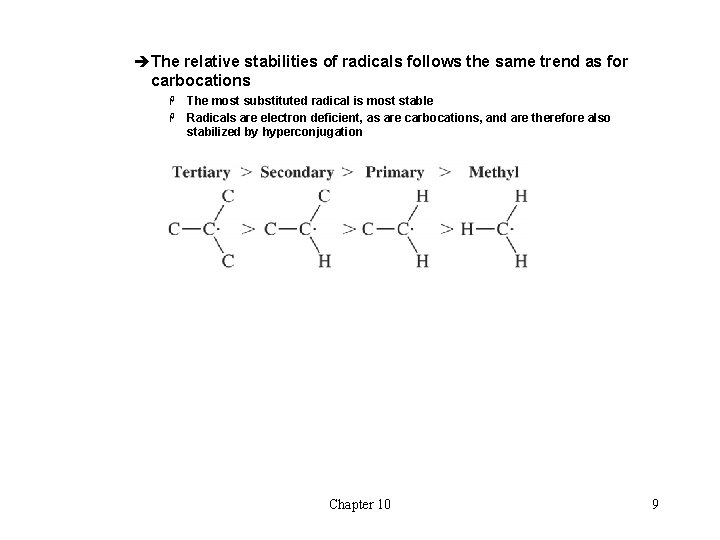
èThe relative stabilities of radicals follows the same trend as for carbocations The most substituted radical is most stable H Radicals are electron deficient, as are carbocations, and are therefore also stabilized by hyperconjugation H Chapter 10 9

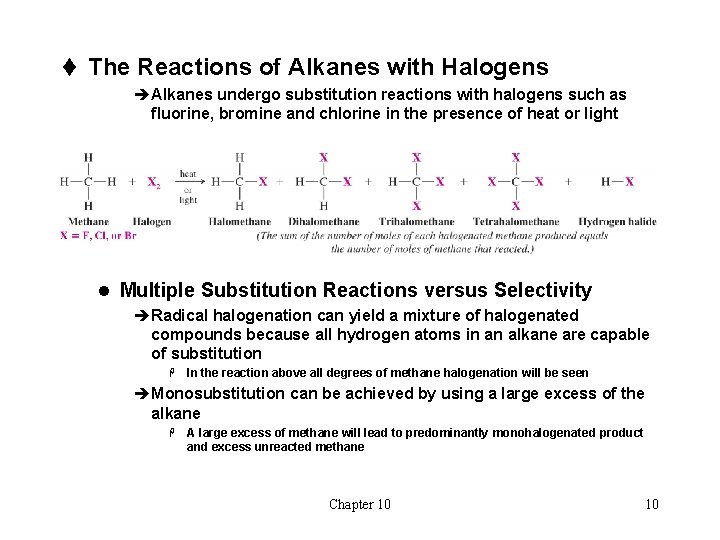
t The Reactions of Alkanes with Halogens èAlkanes undergo substitution reactions with halogens such as fluorine, bromine and chlorine in the presence of heat or light l Multiple Substitution Reactions versus Selectivity èRadical halogenation can yield a mixture of halogenated compounds because all hydrogen atoms in an alkane are capable of substitution H In the reaction above all degrees of methane halogenation will be seen èMonosubstitution can be achieved by using a large excess of the alkane H A large excess of methane will lead to predominantly monohalogenated product and excess unreacted methane Chapter 10 10

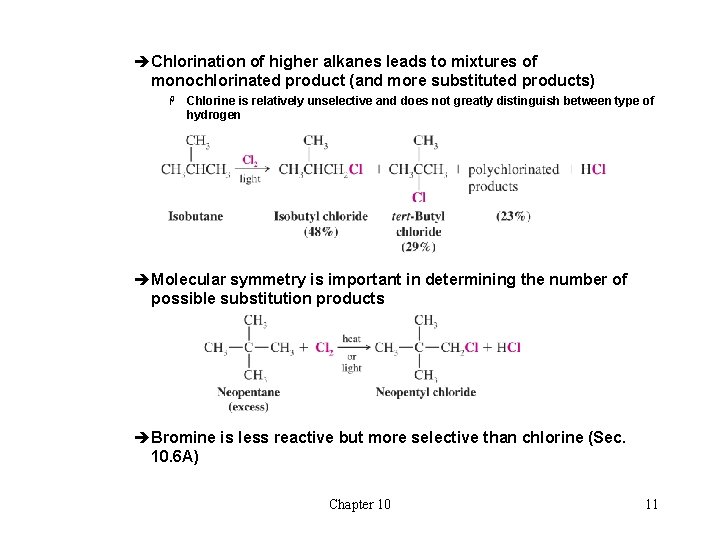
èChlorination of higher alkanes leads to mixtures of monochlorinated product (and more substituted products) H Chlorine is relatively unselective and does not greatly distinguish between type of hydrogen èMolecular symmetry is important in determining the number of possible substitution products èBromine is less reactive but more selective than chlorine (Sec. 10. 6 A) Chapter 10 11

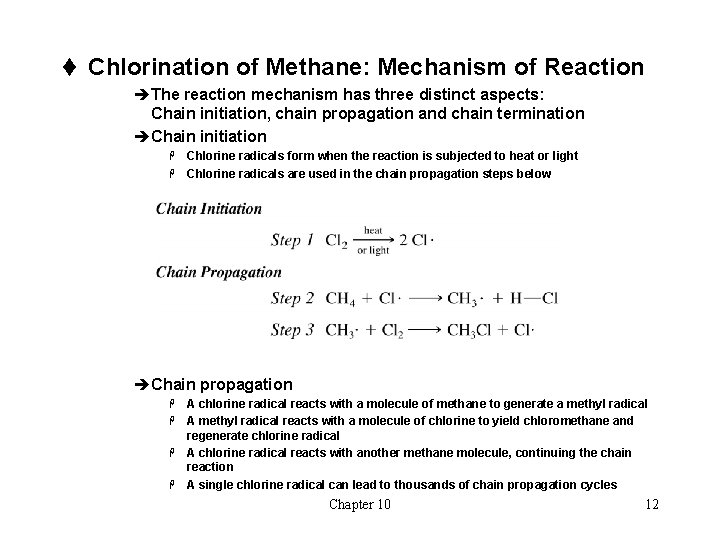
t Chlorination of Methane: Mechanism of Reaction èThe reaction mechanism has three distinct aspects: Chain initiation, chain propagation and chain termination èChain initiation Chlorine radicals form when the reaction is subjected to heat or light H Chlorine radicals are used in the chain propagation steps below H èChain propagation A chlorine radical reacts with a molecule of methane to generate a methyl radical H A methyl radical reacts with a molecule of chlorine to yield chloromethane and regenerate chlorine radical H A chlorine radical reacts with another methane molecule, continuing the chain reaction H A single chlorine radical can lead to thousands of chain propagation cycles H Chapter 10 12

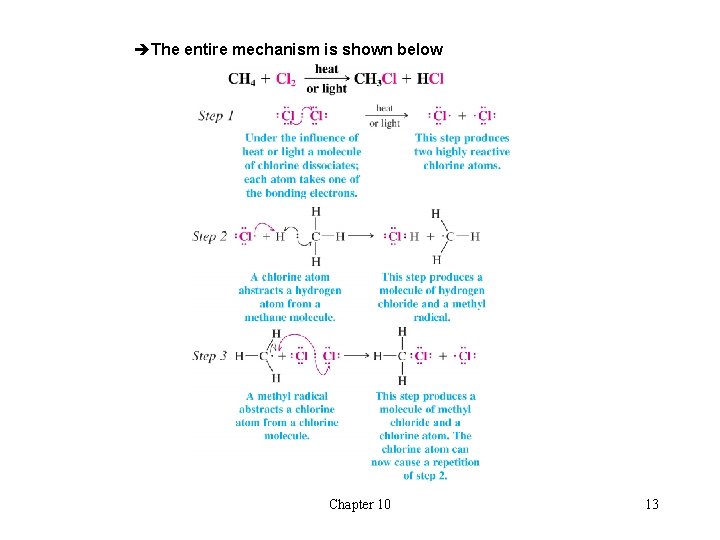
èThe entire mechanism is shown below Chapter 10 13

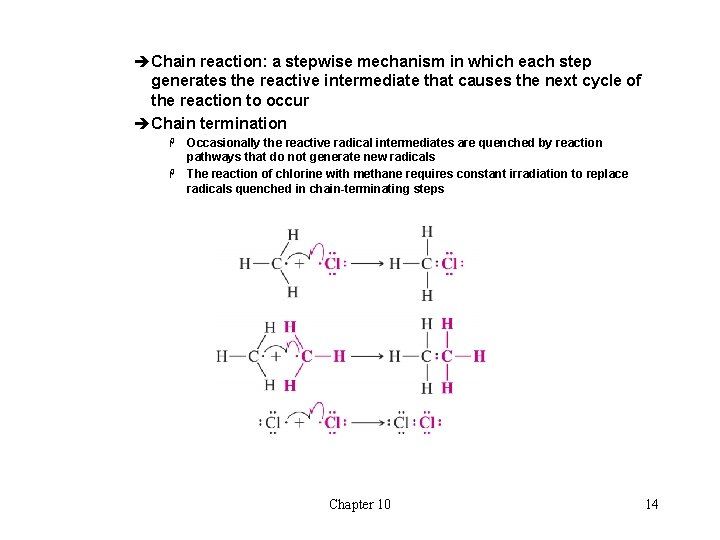
èChain reaction: a stepwise mechanism in which each step generates the reactive intermediate that causes the next cycle of the reaction to occur èChain termination Occasionally the reactive radical intermediates are quenched by reaction pathways that do not generate new radicals H The reaction of chlorine with methane requires constant irradiation to replace radicals quenched in chain-terminating steps H Chapter 10 14

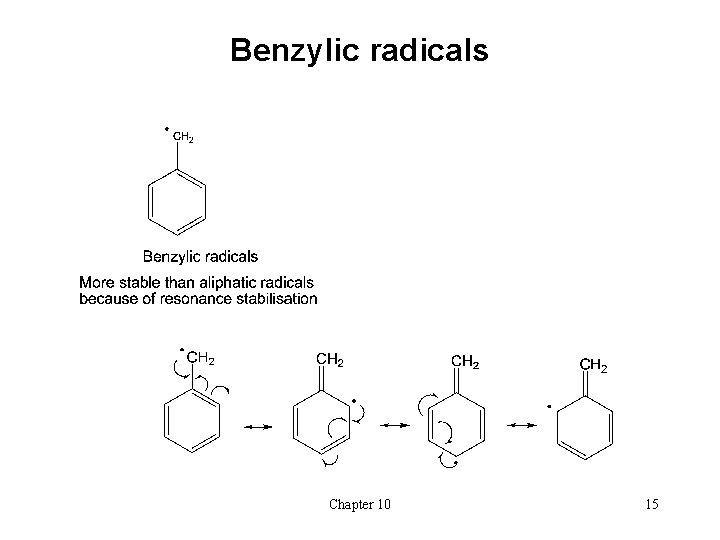
Benzylic radicals Chapter 10 15

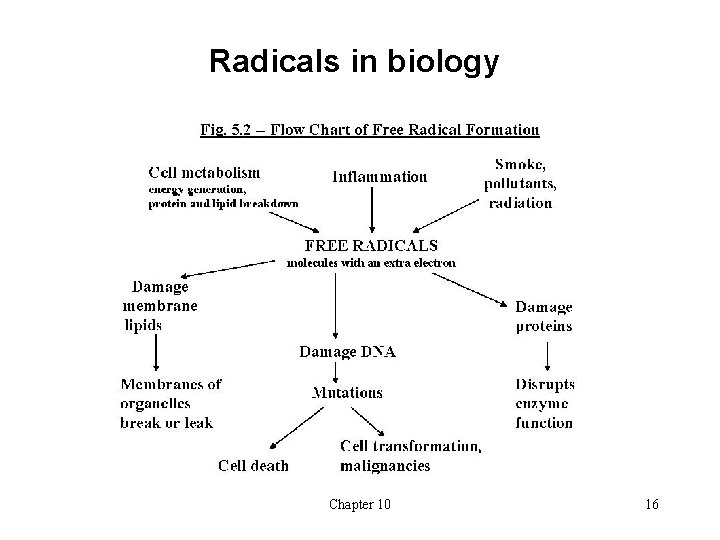
Radicals in biology Chapter 10 16

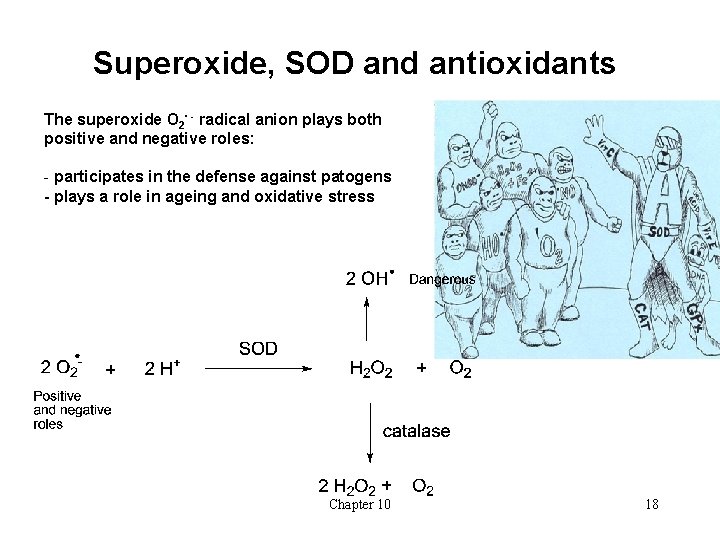
Radicals in biology • Can be formed from • Oxidative metabolic processes in the mitochondria • Irradiation: UV from the sun, X-rays • Toxins in bacteria or fungus • carcinogens in food • Some important biological examples: • The NO∙ radical: involved in e. g. blood pressure regulation, stroke, nerve signals • The superoxide radical anion O 2∙ - - plays a role in ageing • It is formed from oxygen in the body and is formed when molecular oxygen accepts an electron, e. g. from other free radicals • Special enzyme for elimination of O 2∙ - : Superoxide dismutase (SOD) Chapter 10 17

Superoxide, SOD and antioxidants The superoxide O 2∙ - radical anion plays both positive and negative roles: - participates in the defense against patogens - plays a role in ageing and oxidative stress Chapter 10 18

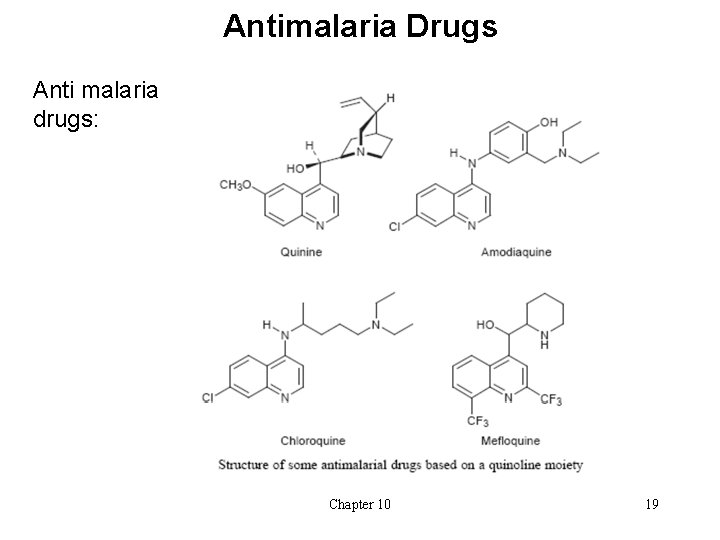
Antimalaria Drugs Anti malaria drugs: Chapter 10 19

Drugs based on radical reactions Anti malaria drugs: Chapter 10 20

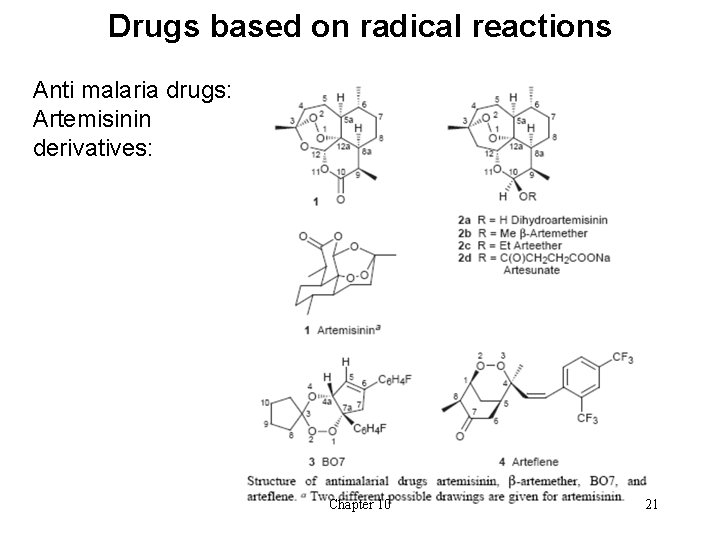
Drugs based on radical reactions Anti malaria drugs: Artemisinin derivatives: Chapter 10 21

Jeg forventer at dere behersker t t t Grunnleggende kunnskap om radikaler: l Hva de er – definisjon l Egenskaper l Hvordan de oppstår l Hvordan de reagerer l Stabilitet l Energibetraktninger l Grunnleggende mekansisme for halogenering av metan Grunnleggende kunnskap om radikaler i biologien Eksempel på radikal-baserte legemidler Chapter 10 22
 Differentiate like radicals from unlike radicals
Differentiate like radicals from unlike radicals What is an entire radical
What is an entire radical Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Oxidation half reaction
Oxidation half reaction Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemistry unit 5 reactions balancing reactions worksheet
Chemistry unit 5 reactions balancing reactions worksheet Entire radical form
Entire radical form Unit 6 radical functions homework 4 rational exponents
Unit 6 radical functions homework 4 rational exponents Entire radical form
Entire radical form Universidad alejandro humboldt carreras pensum
Universidad alejandro humboldt carreras pensum Pensum kapitalforvaltning
Pensum kapitalforvaltning Pensum procesos gerenciales
Pensum procesos gerenciales Statskundskab au
Statskundskab au 9 klasse pensum
9 klasse pensum Jus1111 pensum
Jus1111 pensum Plan de estudios ingenieria industrial javeriana
Plan de estudios ingenieria industrial javeriana Chapter 10 chemical reactions
Chapter 10 chemical reactions Chapter 9 chemical reactions test answers
Chapter 9 chemical reactions test answers Adding subtracting and multiplying radical expressions
Adding subtracting and multiplying radical expressions How to simplify square root of 72
How to simplify square root of 72 Radicals and complex numbers
Radicals and complex numbers Equations with rational exponents
Equations with rational exponents