11 1 Free Radicals Free radicals form when





- Slides: 33

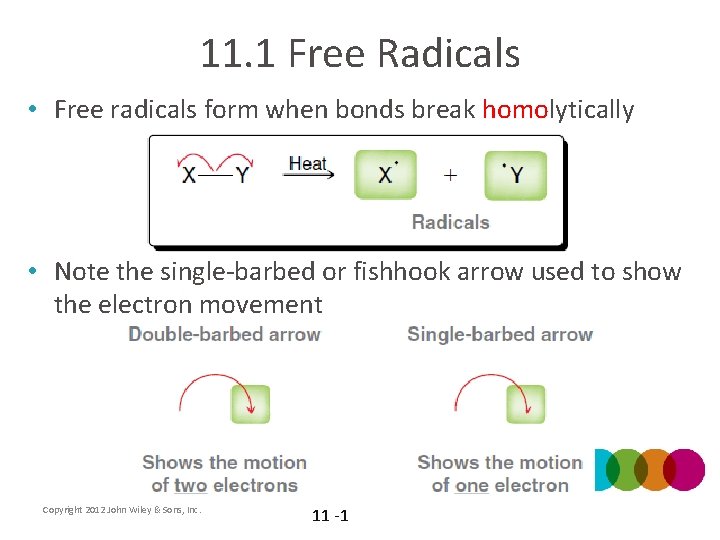
11. 1 Free Radicals • Free radicals form when bonds break homolytically • Note the single-barbed or fishhook arrow used to show the electron movement Copyright 2012 John Wiley & Sons, Inc. 11 -1

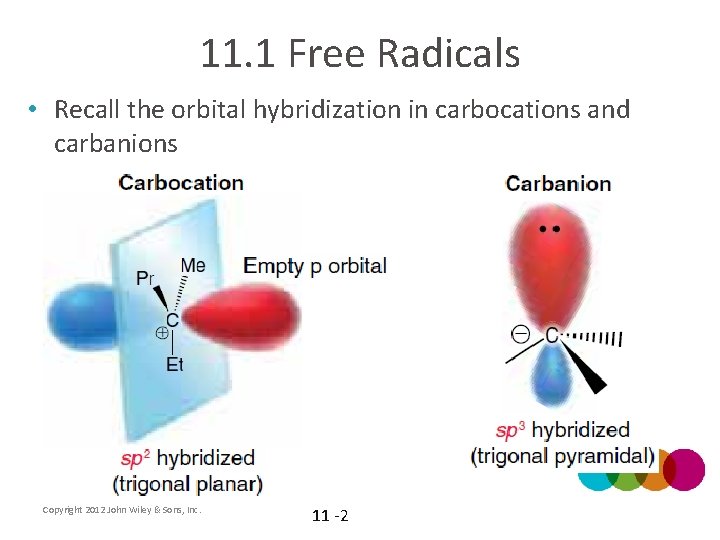
11. 1 Free Radicals • Recall the orbital hybridization in carbocations and carbanions Copyright 2012 John Wiley & Sons, Inc. 11 -2

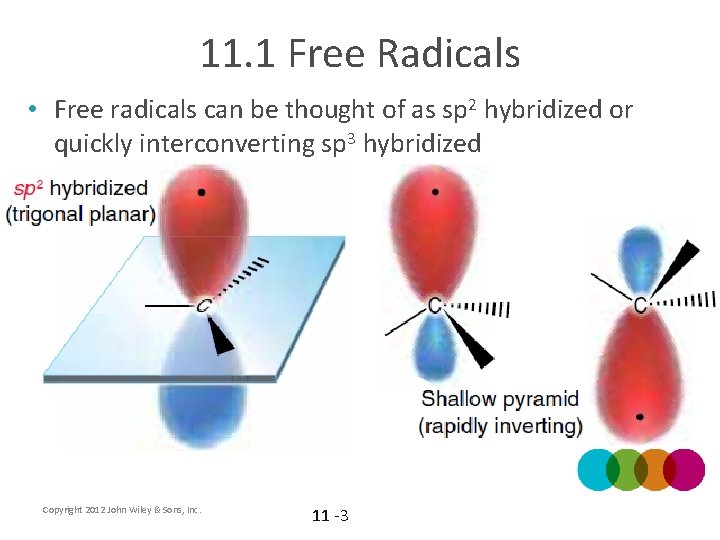
11. 1 Free Radicals • Free radicals can be thought of as sp 2 hybridized or quickly interconverting sp 3 hybridized Copyright 2012 John Wiley & Sons, Inc. 11 -3

11. 1 Free Radical Stability • Free radicals do not have a formal charge but are unstable because of an incomplete octet • Groups that can push (donate) electrons toward the free radical will help to stabilize it. WHY? HOW? Consider hyperconjugation Copyright 2012 John Wiley & Sons, Inc. 11 -4

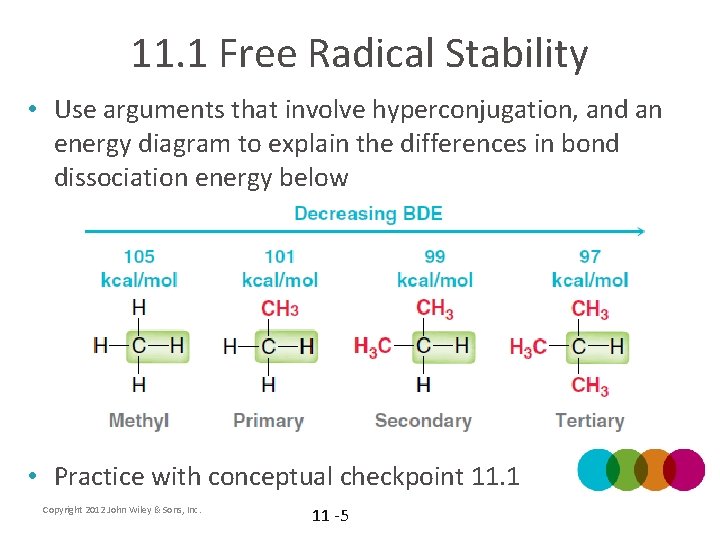
11. 1 Free Radical Stability • Use arguments that involve hyperconjugation, and an energy diagram to explain the differences in bond dissociation energy below • Practice with conceptual checkpoint 11. 1 Copyright 2012 John Wiley & Sons, Inc. 11 -5

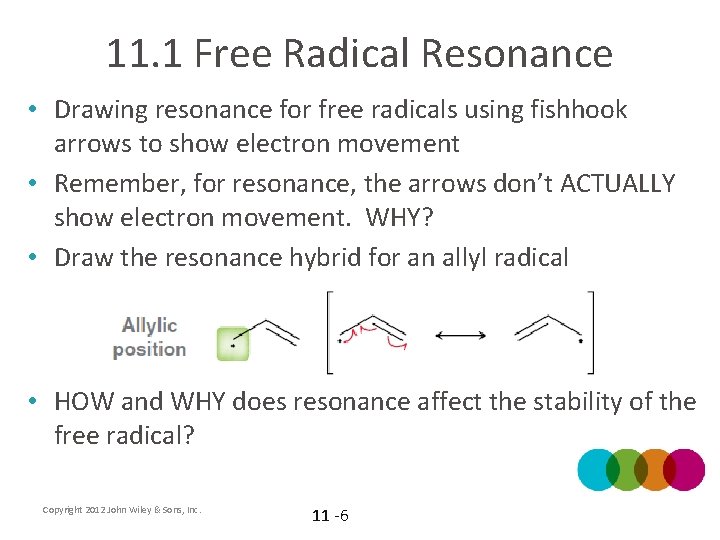
11. 1 Free Radical Resonance • Drawing resonance for free radicals using fishhook arrows to show electron movement • Remember, for resonance, the arrows don’t ACTUALLY show electron movement. WHY? • Draw the resonance hybrid for an allyl radical • HOW and WHY does resonance affect the stability of the free radical? Copyright 2012 John Wiley & Sons, Inc. 11 -6

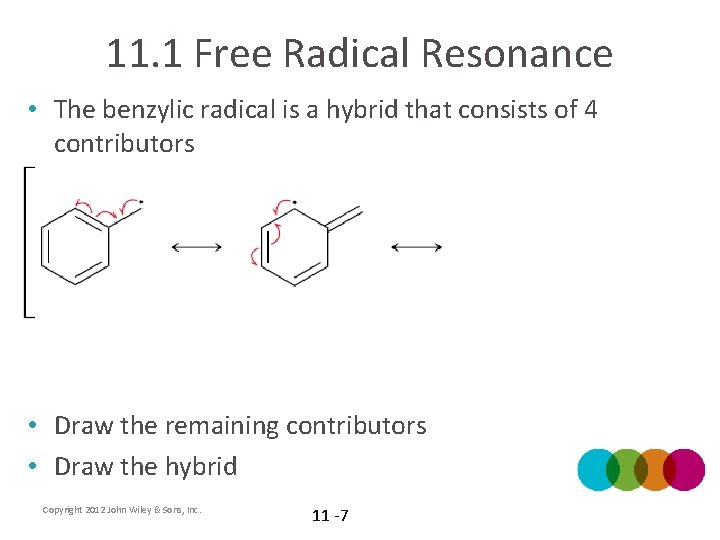
11. 1 Free Radical Resonance • The benzylic radical is a hybrid that consists of 4 contributors • Draw the remaining contributors • Draw the hybrid Copyright 2012 John Wiley & Sons, Inc. 11 -7

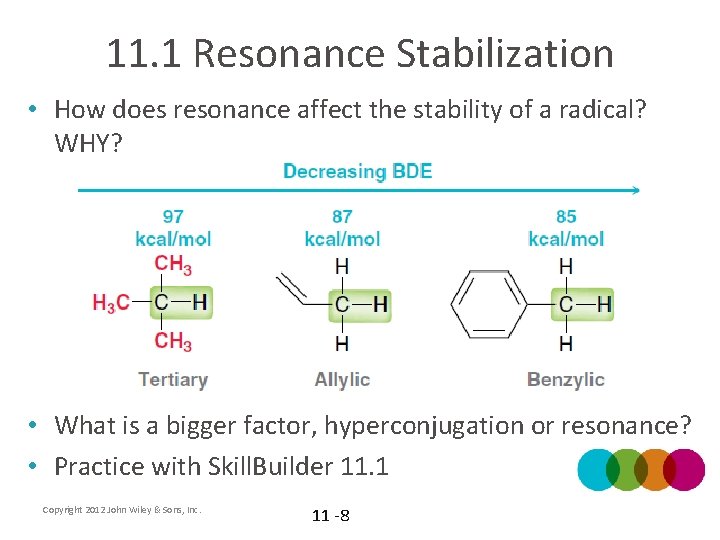
11. 1 Resonance Stabilization • How does resonance affect the stability of a radical? WHY? • What is a bigger factor, hyperconjugation or resonance? • Practice with Skill. Builder 11. 1 Copyright 2012 John Wiley & Sons, Inc. 11 -8

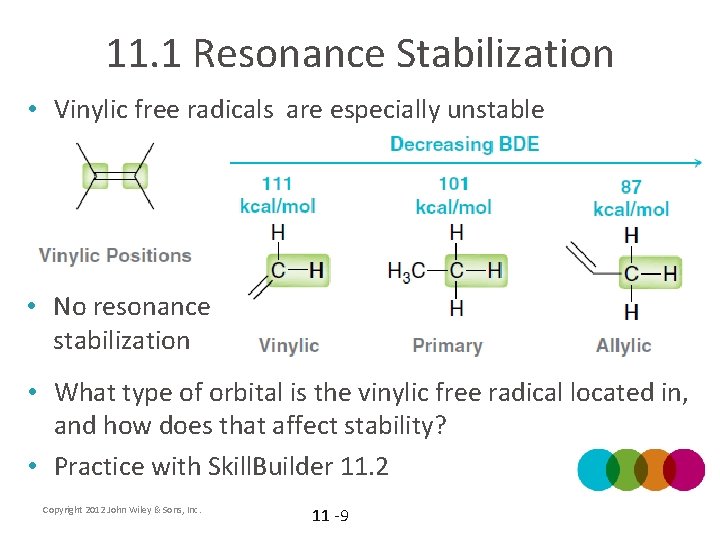
11. 1 Resonance Stabilization • Vinylic free radicals are especially unstable • No resonance stabilization • What type of orbital is the vinylic free radical located in, and how does that affect stability? • Practice with Skill. Builder 11. 2 Copyright 2012 John Wiley & Sons, Inc. 11 -9

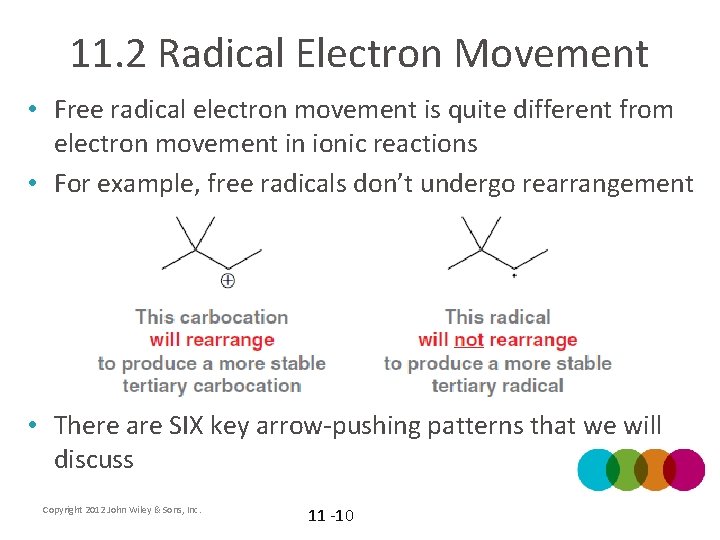
11. 2 Radical Electron Movement • Free radical electron movement is quite different from electron movement in ionic reactions • For example, free radicals don’t undergo rearrangement • There are SIX key arrow-pushing patterns that we will discuss Copyright 2012 John Wiley & Sons, Inc. 11 -10

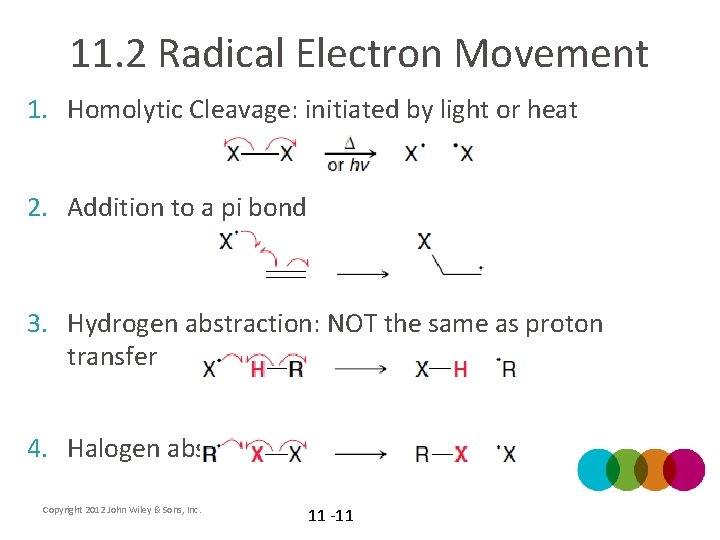
11. 2 Radical Electron Movement 1. Homolytic Cleavage: initiated by light or heat 2. Addition to a pi bond 3. Hydrogen abstraction: NOT the same as proton transfer 4. Halogen abstraction Copyright 2012 John Wiley & Sons, Inc. 11 -11

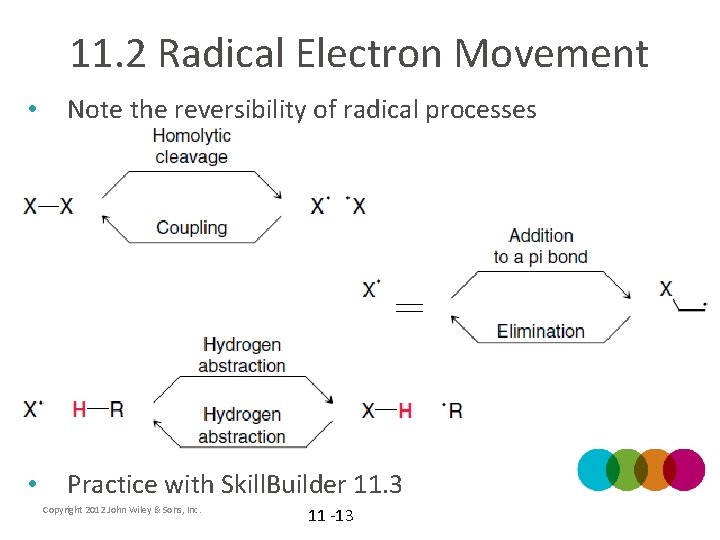
11. 2 Radical Electron Movement 5. Elimination: the radical from the α carbon is pushed toward the β carbon to eliminate a group on the β carbon (reverse of addition to a pi bond) – The –X group is NOT a leaving group. WHY? 6. Coupling: the reverse of homolytic cleavage • Note that radical electron movement generally involves 2 or 3 fishhook arrows Copyright 2012 John Wiley & Sons, Inc. 11 -12

11. 2 Radical Electron Movement • Note the reversibility of radical processes • Practice with Skill. Builder 11. 3 Copyright 2012 John Wiley & Sons, Inc. 11 -13

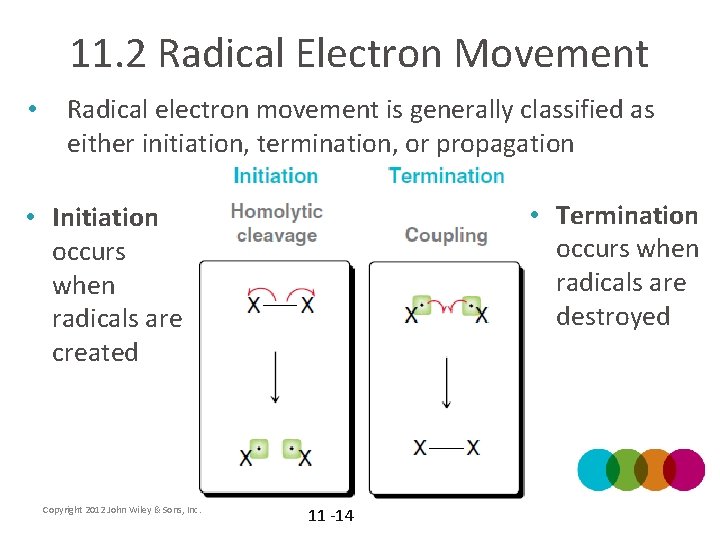
11. 2 Radical Electron Movement • Radical electron movement is generally classified as either initiation, termination, or propagation • Termination occurs when radicals are destroyed • Initiation occurs when radicals are created Copyright 2012 John Wiley & Sons, Inc. 11 -14

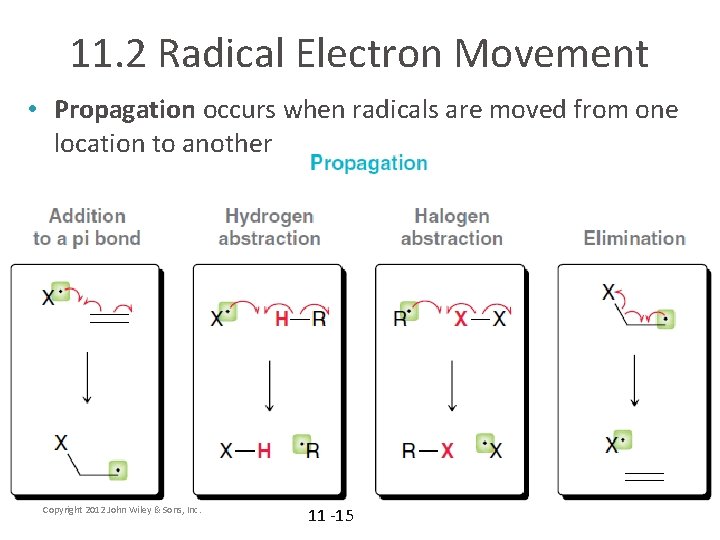
11. 2 Radical Electron Movement • Propagation occurs when radicals are moved from one location to another Copyright 2012 John Wiley & Sons, Inc. 11 -15

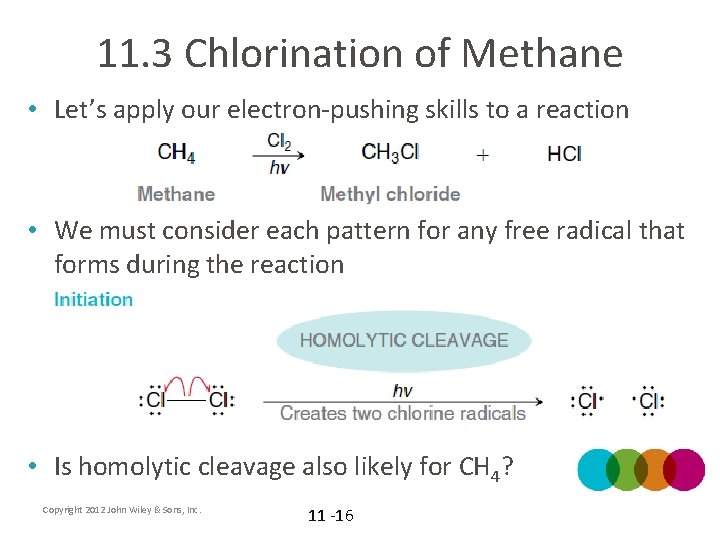
11. 3 Chlorination of Methane • Let’s apply our electron-pushing skills to a reaction • We must consider each pattern for any free radical that forms during the reaction • Is homolytic cleavage also likely for CH 4? Copyright 2012 John Wiley & Sons, Inc. 11 -16

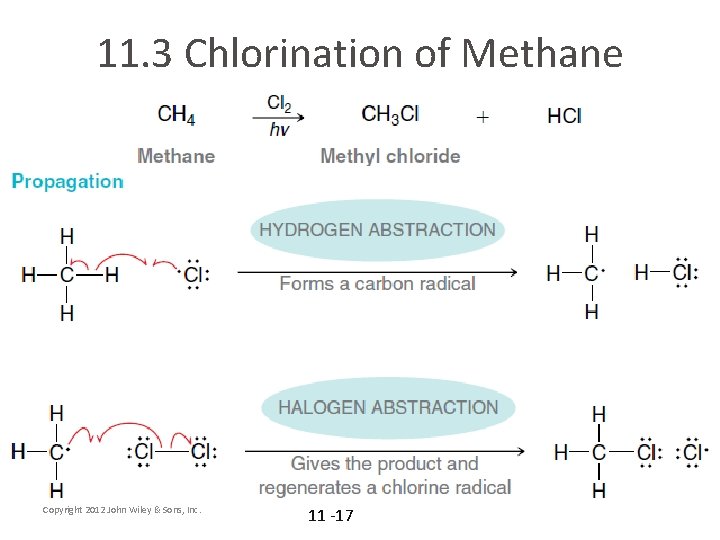
11. 3 Chlorination of Methane Copyright 2012 John Wiley & Sons, Inc. 11 -17

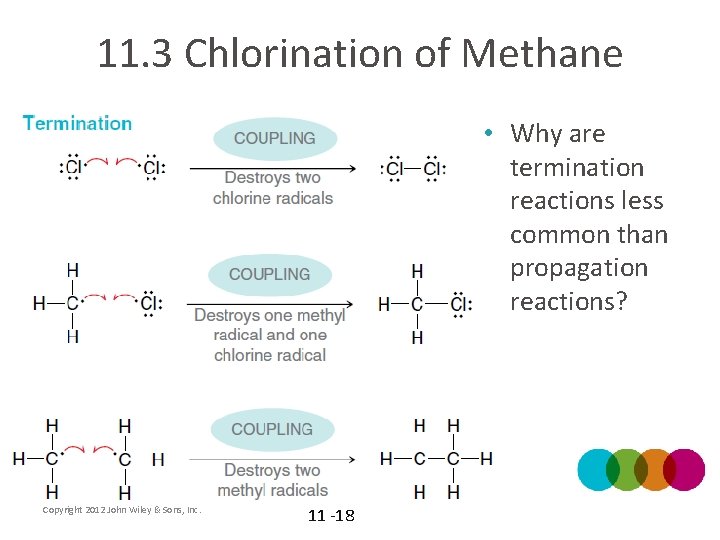
11. 3 Chlorination of Methane • Why are termination reactions less common than propagation reactions? Copyright 2012 John Wiley & Sons, Inc. 11 -18

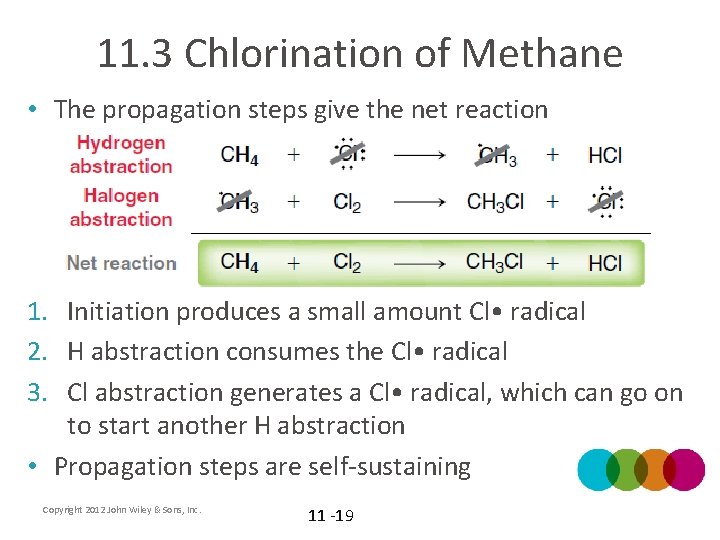
11. 3 Chlorination of Methane • The propagation steps give the net reaction 1. Initiation produces a small amount Cl • radical 2. H abstraction consumes the Cl • radical 3. Cl abstraction generates a Cl • radical, which can go on to start another H abstraction • Propagation steps are self-sustaining Copyright 2012 John Wiley & Sons, Inc. 11 -19

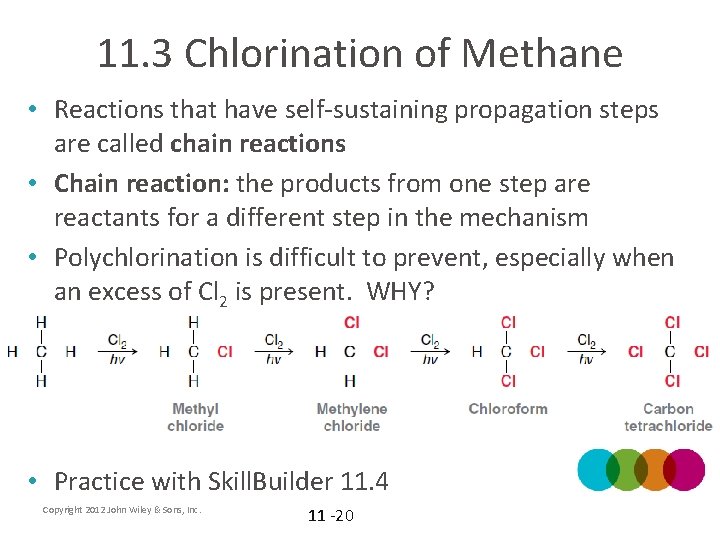
11. 3 Chlorination of Methane • Reactions that have self-sustaining propagation steps are called chain reactions • Chain reaction: the products from one step are reactants for a different step in the mechanism • Polychlorination is difficult to prevent, especially when an excess of Cl 2 is present. WHY? • Practice with Skill. Builder 11. 4 Copyright 2012 John Wiley & Sons, Inc. 11 -20

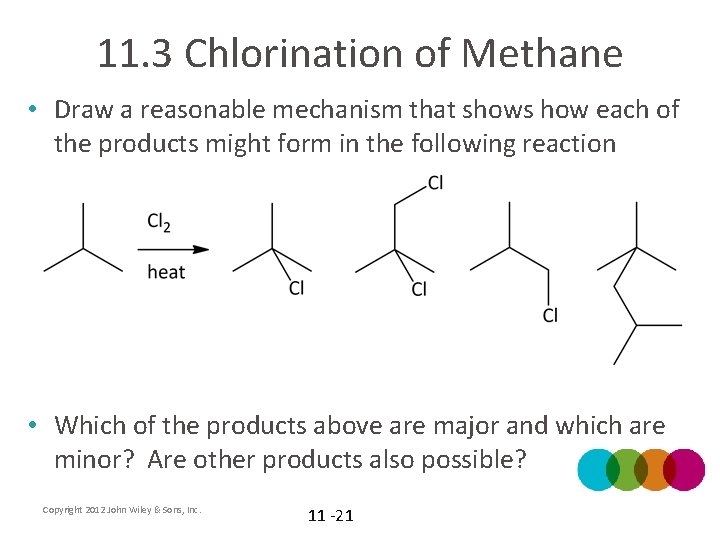
11. 3 Chlorination of Methane • Draw a reasonable mechanism that shows how each of the products might form in the following reaction • Which of the products above are major and which are minor? Are other products also possible? Copyright 2012 John Wiley & Sons, Inc. 11 -21

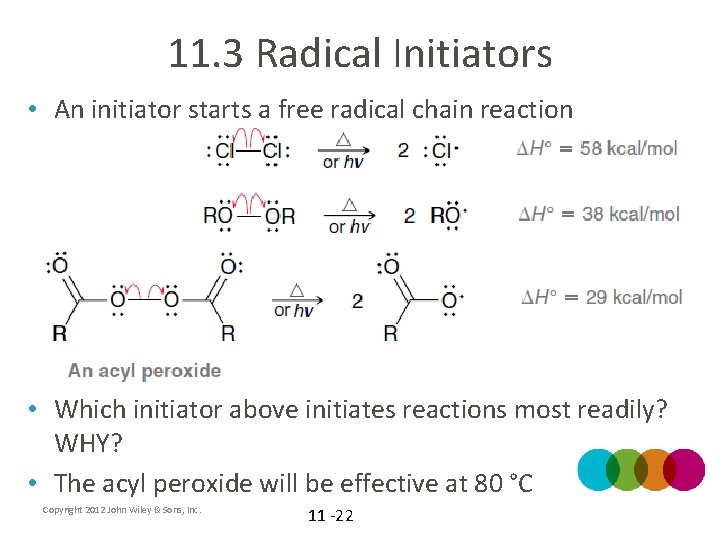
11. 3 Radical Initiators • An initiator starts a free radical chain reaction • Which initiator above initiates reactions most readily? WHY? • The acyl peroxide will be effective at 80 °C Copyright 2012 John Wiley & Sons, Inc. 11 -22

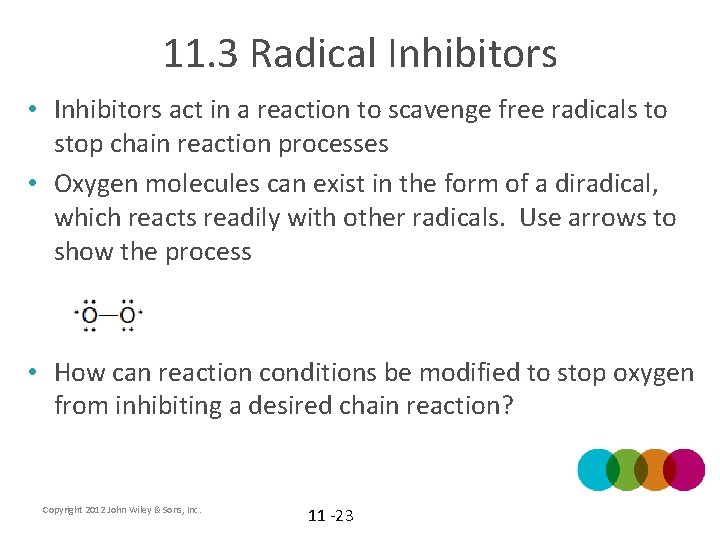
11. 3 Radical Inhibitors • Inhibitors act in a reaction to scavenge free radicals to stop chain reaction processes • Oxygen molecules can exist in the form of a diradical, which reacts readily with other radicals. Use arrows to show the process • How can reaction conditions be modified to stop oxygen from inhibiting a desired chain reaction? Copyright 2012 John Wiley & Sons, Inc. 11 -23

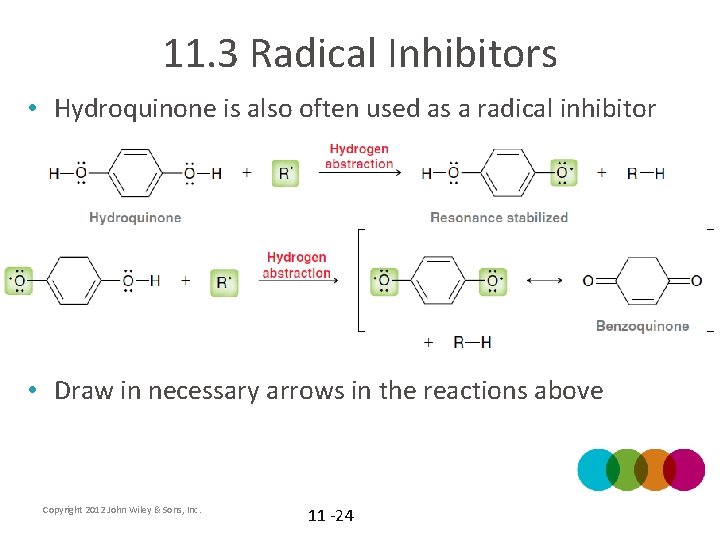
11. 3 Radical Inhibitors • Hydroquinone is also often used as a radical inhibitor • Draw in necessary arrows in the reactions above Copyright 2012 John Wiley & Sons, Inc. 11 -24

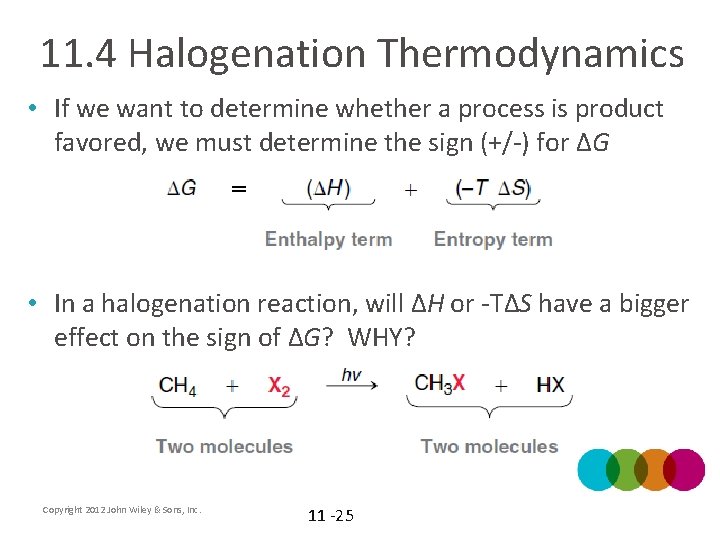
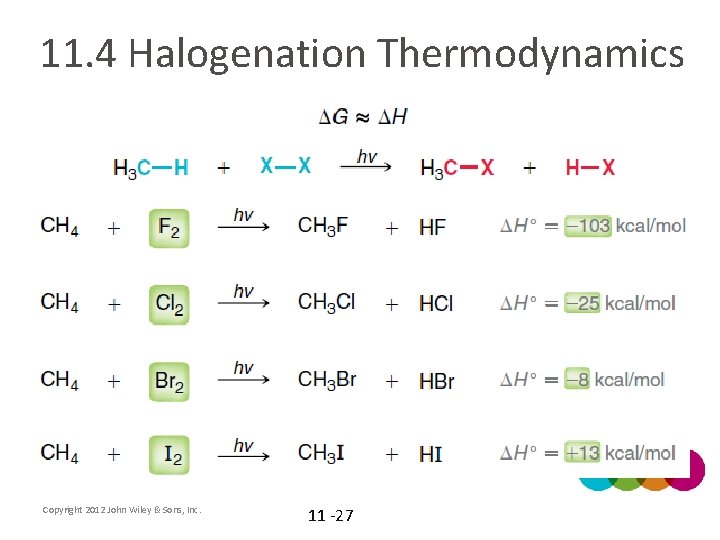
11. 4 Halogenation Thermodynamics • If we want to determine whether a process is product favored, we must determine the sign (+/-) for ΔG • In a halogenation reaction, will ΔH or -TΔS have a bigger effect on the sign of ΔG? WHY? Copyright 2012 John Wiley & Sons, Inc. 11 -25

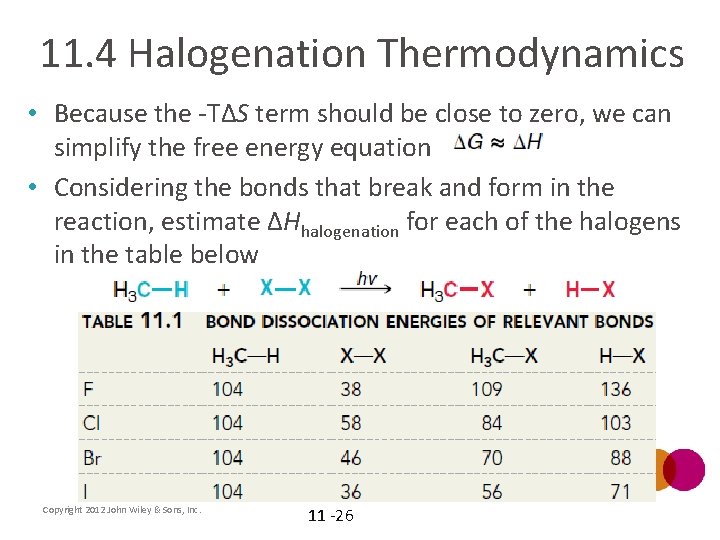
11. 4 Halogenation Thermodynamics • Because the -TΔS term should be close to zero, we can simplify the free energy equation • Considering the bonds that break and form in the reaction, estimate ΔHhalogenation for each of the halogens in the table below Copyright 2012 John Wiley & Sons, Inc. 11 -26

11. 4 Halogenation Thermodynamics Copyright 2012 John Wiley & Sons, Inc. 11 -27

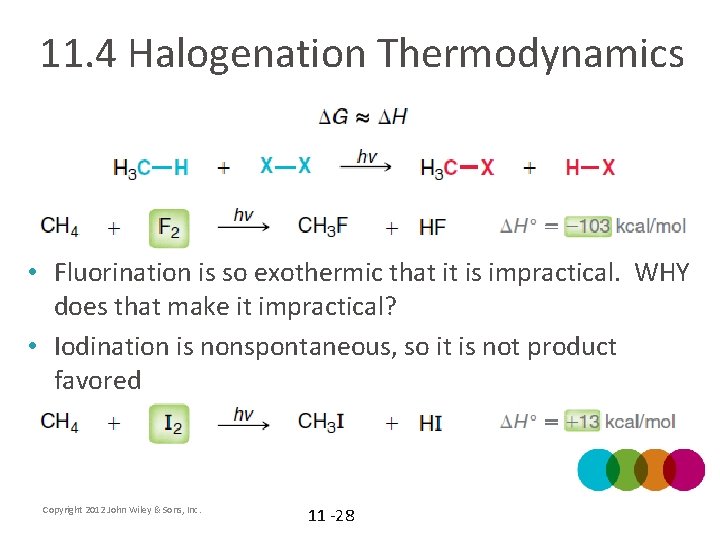
11. 4 Halogenation Thermodynamics • Fluorination is so exothermic that it is impractical. WHY does that make it impractical? • Iodination is nonspontaneous, so it is not product favored Copyright 2012 John Wiley & Sons, Inc. 11 -28

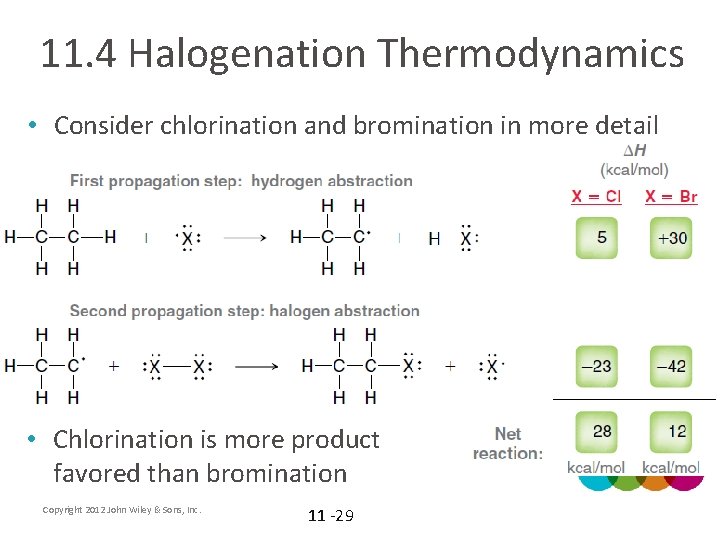
11. 4 Halogenation Thermodynamics • Consider chlorination and bromination in more detail • Chlorination is more product favored than bromination Copyright 2012 John Wiley & Sons, Inc. 11 -29

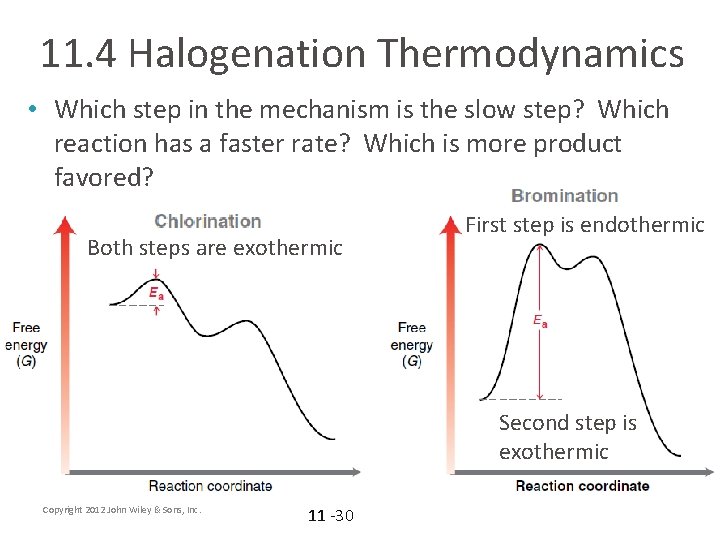
11. 4 Halogenation Thermodynamics • Which step in the mechanism is the slow step? Which reaction has a faster rate? Which is more product favored? Both steps are exothermic First step is endothermic Second step is exothermic Copyright 2012 John Wiley & Sons, Inc. 11 -30

Study Guide for Sections 11. 1 -11. 4 DAY 1, Terms to know: Sections 11. 1 -11. 4 Free radical, homolytic, hyperconjugation, benzylic, vinylic, hydrogen abstraction, halogen abstraction, coupling, initiation, propagation, termination, chain reaction, radical halogenation, inhibitors DAY 1, Specific outcomes and skills that may be tested on exam 1: Sections 11. 1 -11. 4 • Be able to predict whether homolytic cleavage is exo or endo thermic. • Be able to use fishhook arrows to show correct movement of electrons in radical reactions • Be able to describe the geometry and hybridization of free radical carbons • Be able to determine relative stabilities of radicals based on resonance and hyperconjugation • Be able to draw radical resonance contributors and a resonance hybrid • Be able to explain why vinylic free radicals are especially unstable • Be able to draw and label initiation, propagation, and termination steps in a radical mechanism • Be able to predict major products and give a mechanism for the free radical halogenations of a hydrocarbon • Be able to draw the structure of a radical inhibitor and explain mechanistically how an inhibitor acts to prevent radical reaction • Be able to give a complete description of thermodynamic analysis of radical halogenations processes with either Cl, Br, or I.

Extra Practice Problems for Sections 11. 1 -11. 4 Complete these problems outside of class until you are confident you have learned the SKILLS in this section outlined on the study guide and we will review some of them next class period. 11. 1 a 11. 2 11. 3 11. 4 11. 5 11. 6 11. 7 11. 8 11. 9 11. 10 d 11. 11 11. 23 11. 24 11. 27

Prep for Day 2 Must Watch videos: https: //www. youtube. com/watch? v=enox. ZMr 7 y 0 s (free radical resonance, Leah) https: //www. youtube. com/watch? v=5 Hgzslt. Ww. K 8 (free radical chlorination, Khan) https: //www. youtube. com/watch? v=LCSOh. Cl. ZNy. I (radical halogenation, Dave) https: //www. youtube. com/watch? v=sh 7 p. Pv. ZKNIM (chlorination versus bromination kinetics, thermodynamics, and stereochem, FLC) https: //www. youtube. com/watch? v=Uf 2 v_-Xj-2 Y (allylic halogenation, FLC) https: //www. youtube. com/watch? v=BRza. Qcm. FLes (autooxidation, JP Mc. Cormick) Other helpful videos: http: //ocw. uci. edu/lectures/chemistry_202_lecture_22_organic_reaction_mechanisms_ii_radical_structur e__reactivity. html (radical mechanisms, UC-Irvine) http: //ocw. uci. edu/lectures/chemistry_202_lecture_23_organic_reaction_mechanisms_ii_radical_reaction s. html (radical reactions, UC-Irvine) Read Sections 11. 1 -11. 9
 Addition radicals examples
Addition radicals examples What is an entire radical
What is an entire radical Present continuous tense interrogative
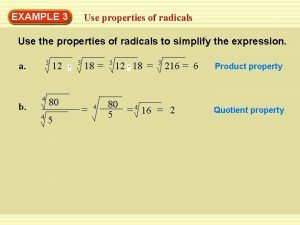
Present continuous tense interrogative Quotient property of radicals
Quotient property of radicals Product property of radicals
Product property of radicals What is the product property of radicals
What is the product property of radicals Solve radical inequalities
Solve radical inequalities Product property of radicals
Product property of radicals Rationalize denominator
Rationalize denominator Simplifying radicals worksheet no variables
Simplifying radicals worksheet no variables Multiplying radicals
Multiplying radicals Index of the radical
Index of the radical Simplify each radical expression
Simplify each radical expression Simplifying radical expressions algebra 1
Simplifying radical expressions algebra 1 Radicals homework
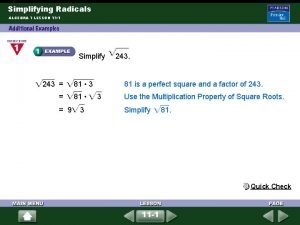
Radicals homework Unit 7-1 simplifying radicals
Unit 7-1 simplifying radicals Simplifying radicals calculator
Simplifying radicals calculator Property of radicals
Property of radicals Adding subtracting and multiplying radical expressions
Adding subtracting and multiplying radical expressions Fraction exponents
Fraction exponents Parts of radical sign
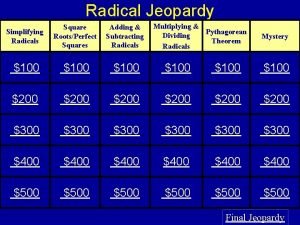
Parts of radical sign Simplifying radicals jeopardy
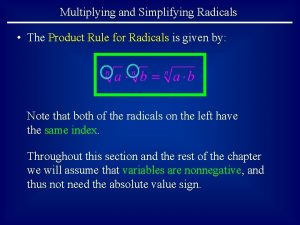
Simplifying radicals jeopardy What is the product rule for radicals
What is the product rule for radicals Quotient property of radicals
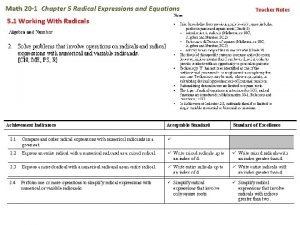
Quotient property of radicals Lesson 20-1 radical expressions
Lesson 20-1 radical expressions Properties of radicals
Properties of radicals Conjugate of a cube root
Conjugate of a cube root Simplify
Simplify How to divide radicals by whole numbers
How to divide radicals by whole numbers Dmca square root
Dmca square root Simplifying radical quiz
Simplifying radical quiz Inverse pythagoras theorem
Inverse pythagoras theorem Radical inequalities
Radical inequalities Radical and irrational numbers
Radical and irrational numbers