Quantum Physics Study Questions PHYS 252 Dr Varriano













- Slides: 16

Quantum Physics Study Questions PHYS 252 Dr. Varriano

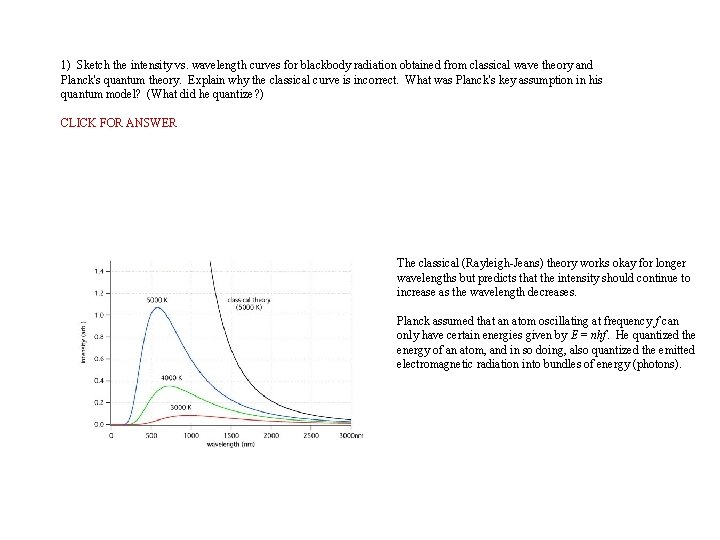
1) Sketch the intensity vs. wavelength curves for blackbody radiation obtained from classical wave theory and Planck's quantum theory. Explain why the classical curve is incorrect. What was Planck's key assumption in his quantum model? (What did he quantize? ) CLICK FOR ANSWER The classical (Rayleigh-Jeans) theory works okay for longer wavelengths but predicts that the intensity should continue to increase as the wavelength decreases. Planck assumed that an atom oscillating at frequency f can only have certain energies given by E = nhf. He quantized the energy of an atom, and in so doing, also quantized the emitted electromagnetic radiation into bundles of energy (photons).

2) What is the photoelectric effect? Give at least two observed characteristics of the photoelectric effect that cannot be explained by the classical wave theory of light. Describe how the photon model explains these characteristics. CLICK FOR ANSWER Light hits material and electron is ejected. (The electron is called a “photoelectron”. ) Observation 1 For frequencies below a certain cut-off frequency, no photoelectrons are seen. Explanation: Since one electron interacts with one photon at a time, the one photon must have sufficient energy (equal to the work function of the material) to eject the electron. The energy of a photon is Eph= hf so there is a minimum frequency. Observation 2 The stopping potential, and therefore the maximum kinetic energy of a photoelectron, is independent of light intensity. Explanation: Since one electron interacts with one photon at a time, the energy of one photon determines the maximum kinetic energy according to Kmax = Eph-. Increasing the intensity increases the number of photons but not the energy of a single photon. Observation 3 A plot of stopping potential versus frequency is linear. Explanation: Since one electron interacts with one photon at a time, the energy of one photon determines the maximum kinetic energy according to the linear equation Kmax = Eph- = hf-. Since Kmax= e Vs, a plot of Vs vs. f is also linear. Observation 4 Photoelectrons are emitted in a very short time even in very low light intensities. Explanation : It only takes one photon to eject an electron.

3) What is Compton scattering? Is Compton scattering explained by the classical wave model of light or by the photon model? CLICK FOR ANSWER Scattering of x-rays off of electrons. Explained by the photon model.

4) List Bohr's four postulates in his model of the Hydrogen atom. What physical quantities are quantized? CLICK FOR ANSWER

5) Describe a physical phenomenon that supports the wave nature of light. Describe a physical phenomenon that supports the particle nature of light. CLICK FOR ANSWER Wave Nature of Light • interference (Young’s double slit, thin film, interferometers) • diffraction Particle Nature of Matter (Photons) • thermal radiation • photoelectric effect • light emission and absorption of atoms • Compton scattering

6) Describe a physical phenomenon that supports the particle nature of matter. Describe a physical phenomenon that supports the wave nature of matter. CLICK FOR ANSWER Particle Nature of Matter • All of Physics I and II ! Objects behaving as regular objects. Wave Nature of Matter • diffraction of electrons • quantum atomic models • quantum tunneling

7) Why aren't we aware of the wave nature of macroscopic objects? CLICK FOR ANSWER Their matter wavelengths are just too small to detect. = h / (mv)

8) Describe the Heisenberg Uncertainty Principle (HUP) either in terms of a simultaneous measurement of position and momentum or in terms of a simultaneous measurement of energy and time. What is it about particles that gives rise to the HUP? CLICK FOR ANSWER

9) Write the one-dimensional time-independent Schroedinger equation. From where does the equation come? CLICK FOR ANSWER

10) Write down an integral expression for the probability of finding a particle traveling in one dimension in the region between x=a and x=b. Write down an integral expression for the normalization condition of a one-dimensional wave function. CLICK FOR ANSWER

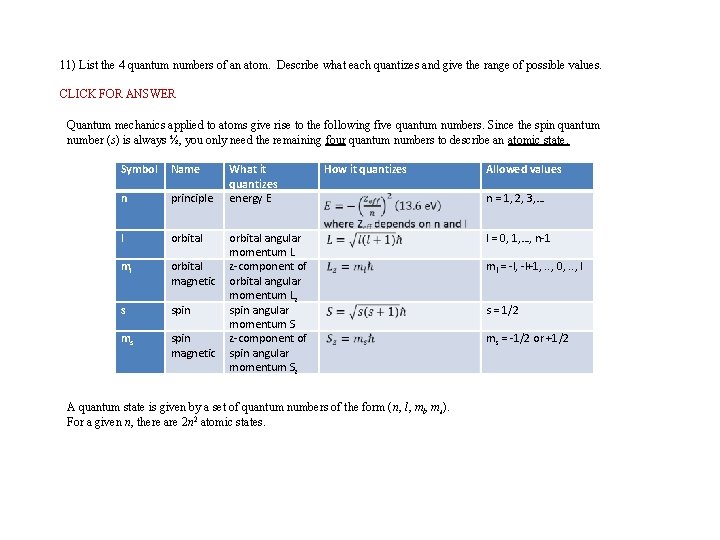
11) List the 4 quantum numbers of an atom. Describe what each quantizes and give the range of possible values. CLICK FOR ANSWER Quantum mechanics applied to atoms give rise to the following five quantum numbers. Since the spin quantum number (s) is always ½, you only need the remaining four quantum numbers to describe an atomic state. Symbol Name n principle l orbital ml s ms What it quantizes energy E How it quantizes orbital angular momentum L orbital z-component of magnetic orbital angular momentum Lz spin angular momentum S spin z-component of magnetic spin angular momentum Sz A quantum state is given by a set of quantum numbers of the form (n, l, ms). For a given n, there are 2 n 2 atomic states. Allowed values n = 1, 2, 3, … l = 0, 1, …, n-1 ml = -l, -l+1, . . , 0, . . , l s = 1/2 ms = -1/2 or +1/2

12) State the Pauli Exclusion Principle. Describe how it helps explain the periodic table of elements. CLICK FOR ANSWER “No two electrons in an atom can have the same set of quantum numbers. ” or “No two electrons in an atom can be in the same state. ” The exclusion principle helps explain the way that orbitals, subshells, and shells are filled with electrons in atoms. This is a major factor in explaining the organization and structure of the periodic table.

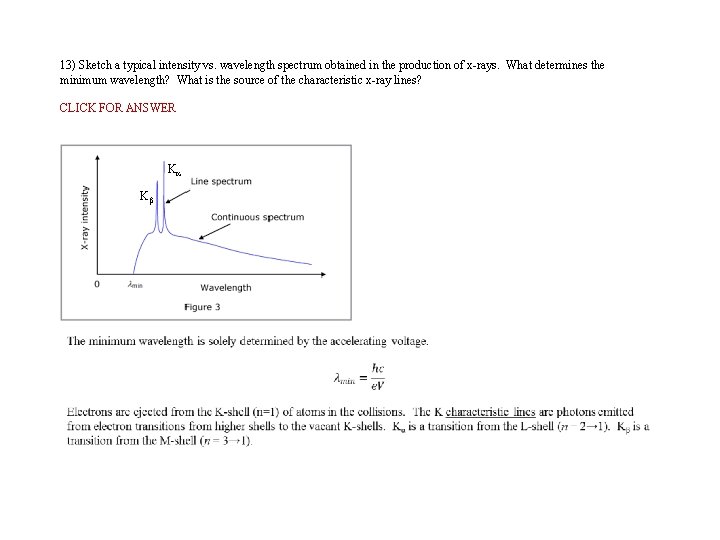
13) Sketch a typical intensity vs. wavelength spectrum obtained in the production of x-rays. What determines the minimum wavelength? What is the source of the characteristic x-ray lines? CLICK FOR ANSWER Ka Kb

14) What are the 3 necessary elements to construct a laser? What does the acronym "laser" stand for? CLICK FOR ANSWER 1. Gain medium – material with atoms that can undergo stimulated emission 2. Excitation Source – gives energy to atom to raise its energy level 3. Mirrors to form a resonating cavity – allows some photons to remain in cavity so to keep stimulating atomic transitions Light Amplification by Stimulated Emission of Radiation

15) Explain how a 3 level laser works. Make sure you know what is meant by a metastable state, population inversion, spontaneous emission, and stimulated emission. CLICK FOR ANSWER 3 1. Pump atom from 1 to 3 using excitation source. 2. Quick decay from 3 to 2. 2 3. Atom “waits” in metastable state for incoming photon*. 1 5. Repeat Step 1. 4. Incoming photon stimulates emission from 2 to 1. metastable state – state where stimulated emission lifetime is shorter than spontaneous emission lifetime, state where stimulated emission is more likely than spontaneous emission population inversion – when there are more atoms in state 2 than in state 1, a necessary condition for continued lasing spontaneous emission– the emission of a photon from the atom due to a transition from a higher to a lower energy level without the aid of another photon stimulated emission– the emission of a photon from the atom due to a transition from a higher to a lower energy level that is caused by the interaction of the atom with an incident photon of the same energy as the emitted photon