Pramlintide Therapy Part 1 of 2 n Pharmacodynamic


- Slides: 9

Pramlintide Therapy Part 1 of 2 n Pharmacodynamic Review n Type 1 Diabetes Efficacy Safety

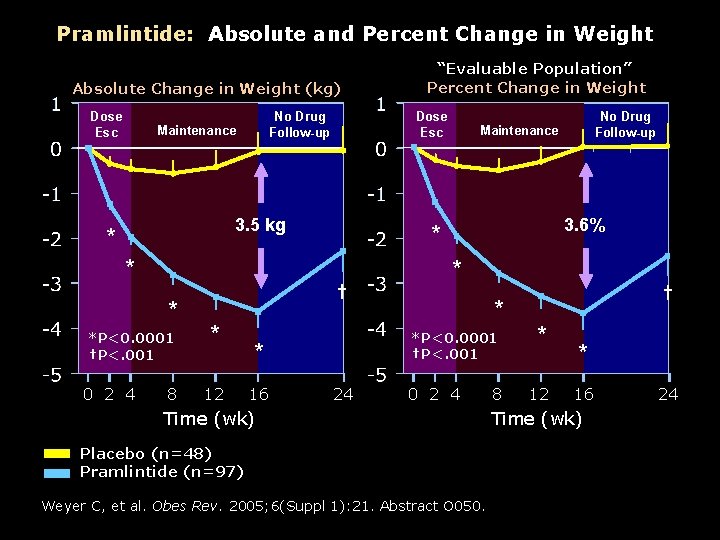
Pramlintide: Absolute and Percent Change in Weight Absolute Change in Weight (kg) Dose Esc No Drug Follow-up Maintenance Dose Esc 3. 5 kg * *P<0. 0001 †P<. 001 0 2 4 8 12 16 3. 6% * 24 † * *P<0. 0001 †P<. 001 * No Drug Follow-up Maintenance * † * “Evaluable Population” Percent Change in Weight 0 2 4 Time (wk) Placebo (n=48) Pramlintide (n=97) Weyer C, et al. Obes Rev. 2005; 6(Suppl 1): 21. Abstract O 050. 8 * 12 * 16 Time (wk) 24

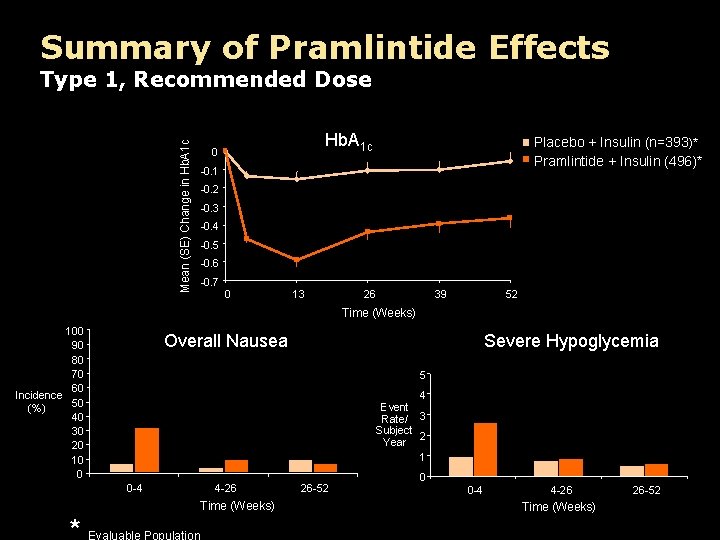
Summary of Pramlintide Effects Mean (SE) Change in Hb. A 1 c Type 1, Recommended Dose Hb. A 1 c 0 Placebo + Insulin (n=393)* Pramlintide + Insulin (496)* -0. 1 -0. 2 -0. 3 -0. 4 -0. 5 -0. 6 -0. 7 0 13 26 39 52 Time (Weeks) 100 90 80 70 60 Incidence 50 (%) 40 30 20 10 0 Overall Nausea Severe Hypoglycemia 5 4 Event Rate/ 3 Subject Year 2 1 0 -4 4 -26 Time (Weeks) 26 -52 0 0 -4 4 -26 Time (Weeks) 26 -52

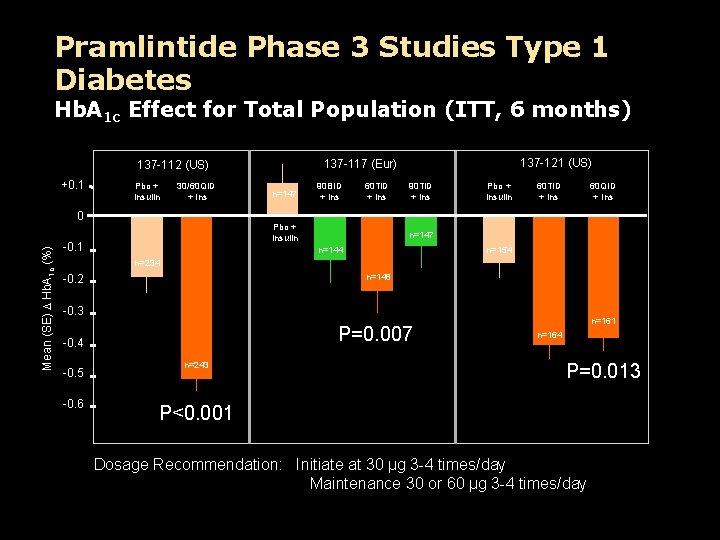
Pramlintide Phase 3 Studies Type 1 Diabetes Hb. A 1 c Effect for Total Population (ITT, 6 months) +0. 1 Pbo + Insulin 30/60 QID + Ins 137 -121 (US) 137 -117 (Eur) 137 -112 (US) n=147 90 BID + Ins 60 TID + Ins 90 TID + Ins Pbo + Insulin 60 TID + Ins 60 QID + Ins Mean (SE) D Hb. A 1 c (%) 0 Pbo + Insulin -0. 1 n=147 n=144 n=154 n=234 -0. 2 n=148 -0. 3 P=0. 007 -0. 4 -0. 5 -0. 6 n=243 n=161 n=164 P=0. 013 P<0. 001 Dosage Recommendation: Initiate at 30 µg 3 -4 times/day Maintenance 30 or 60 µg 3 -4 times/day

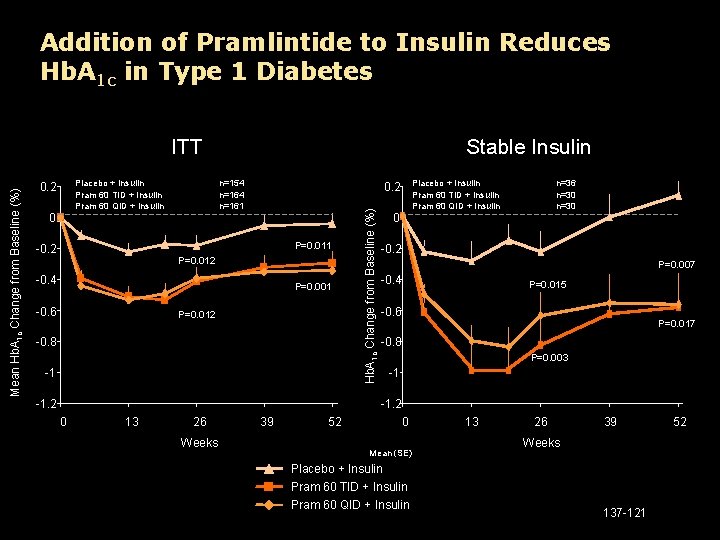
Addition of Pramlintide to Insulin Reduces Hb. A 1 c in Type 1 Diabetes Placebo + Insulin Pram 60 TID + Insulin Pram 60 QID + Insulin 0. 2 0 Stable Insulin n=154 n=161 P=0. 011 -0. 2 P=0. 012 -0. 4 P=0. 001 -0. 6 Placebo + Insulin Pram 60 TID + Insulin Pram 60 QID + Insulin 0. 2 P=0. 012 -0. 8 -1 -1. 2 Hb. A 1 c Change from Baseline (%) Mean Hb. A 1 c Change from Baseline (%) ITT 0 n=36 n=30 -0. 2 P=0. 007 -0. 4 P=0. 015 -0. 6 P=0. 017 -0. 8 P=0. 003 -1 -1. 2 0 13 26 Weeks 39 52 0 Mean (SE) 13 26 39 Weeks Placebo + Insulin Pram 60 TID + Insulin Pram 60 QID + Insulin 137 -121 52

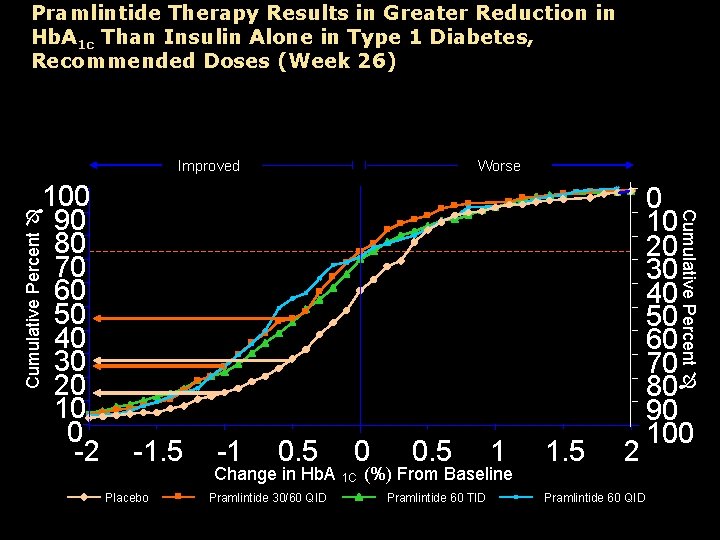
Pramlintide Therapy Results in Greater Reduction in Hb. A 1 c Than Insulin Alone in Type 1 Diabetes, Recommended Doses (Week 26) Improved 0 10 20 30 40 50 60 70 80 90 100 Cumulative Percent 100 90 80 70 60 50 40 30 20 10 0 -2 Worse -1. 5 Placebo -1 0. 5 Change in Hb. A Pramlintide 30/60 QID 0 1 C 0. 5 1 (%) From Baseline Pramlintide 60 TID 1. 5 2 Pramlintide 60 QID

Pramlintide Facilitates Achievement of ADA Targets Type 1 Diabetes, Week 26 Placebo + Insulin Pramlintide Recommended Doses Achieved 8% or Less 28% 47% Achieved 7% or Less 7% 14% Pooled data

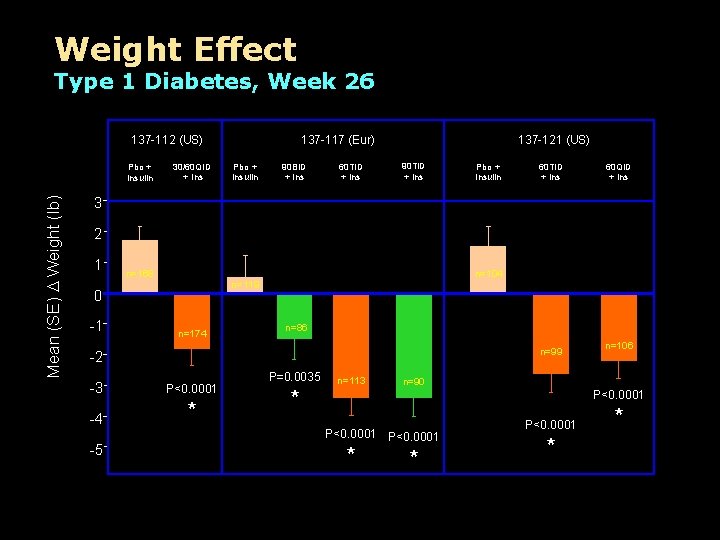
Weight Effect Type 1 Diabetes, Week 26 137 -112 (US) Mean (SE) D Weight (lb) Pbo + Insulin 30/60 QID + Ins 137 -117 (Eur) Pbo + Insulin 90 BID + Ins 60 TID + Ins 137 -121 (US) 90 TID + Ins Pbo + Insulin 60 TID + Ins 60 QID + Ins 3 2 1 n=168 0 -1 n=104 n=119 n=174 n=86 n=99 -2 -3 P<0. 0001 -4 * -5 P=0. 0035 * n=113 n=106 n=90 P<0. 0001 * *

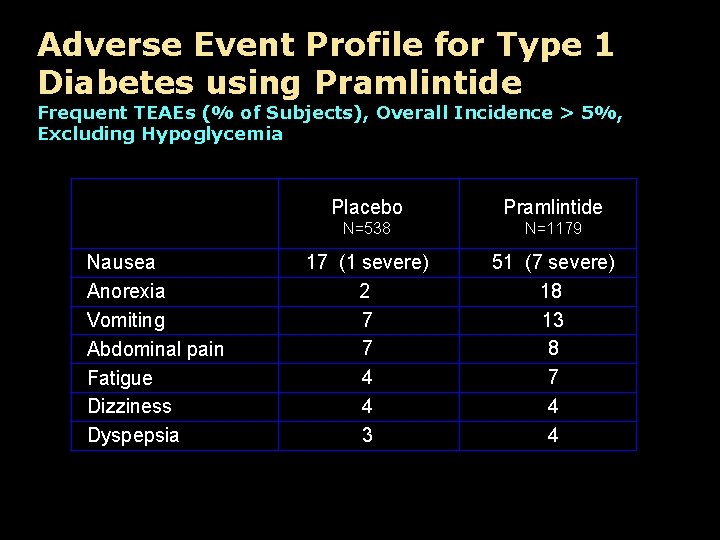
Adverse Event Profile for Type 1 Diabetes using Pramlintide Frequent TEAEs (% of Subjects), Overall Incidence > 5%, Excluding Hypoglycemia Nausea Anorexia Vomiting Abdominal pain Fatigue Dizziness Dyspepsia Placebo Pramlintide N=538 N=1179 17 (1 severe) 2 7 7 4 4 3 51 (7 severe) 18 13 8 7 4 4
 Pharmacodynamic definition
Pharmacodynamic definition Both psychoanalysis and humanistic therapy stress
Both psychoanalysis and humanistic therapy stress Bioness integrated therapy system occupational therapy
Bioness integrated therapy system occupational therapy Humanistic therapies aim to boost
Humanistic therapies aim to boost Family therapy part 2
Family therapy part 2 Ossr physical therapy
Ossr physical therapy The phase of the moon you see depends on ______.
The phase of the moon you see depends on ______. Brainpop ratios
Brainpop ratios Minitab adalah
Minitab adalah Technical description examples
Technical description examples