Pramlintide An analog of amylin that overcomes the


- Slides: 10

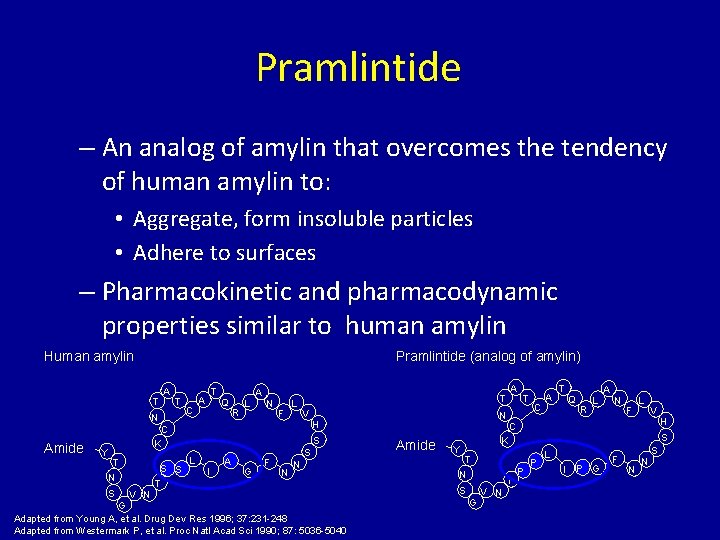
Pramlintide – An analog of amylin that overcomes the tendency of human amylin to: • Aggregate, form insoluble particles • Adhere to surfaces – Pharmacokinetic and pharmacodynamic properties similar to human amylin Human amylin Pramlintide (analog of amylin) T A T N C T A Q R L A N F L V Y S K T N S V N S S T L I A G F S N N G Adapted from Young A, et al. Drug Dev Res 1996; 37: 231 -248 Adapted from Westermark P, et al. Proc Natl Acad Sci 1990; 87: 5036 -5040 T N H C Amide A T C A T Q R L A N F L V H C Amide Y S K T N S G V N T P P L I P G F N N S

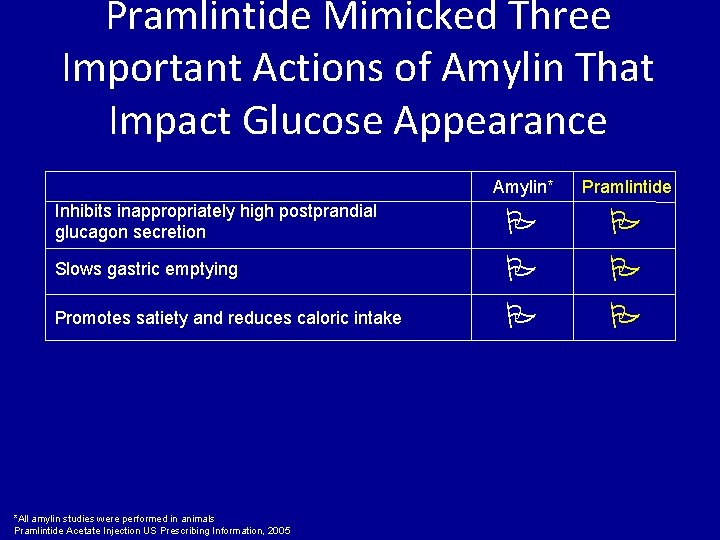
Pramlintide Mimicked Three Important Actions of Amylin That Impact Glucose Appearance Inhibits inappropriately high postprandial glucagon secretion Slows gastric emptying Promotes satiety and reduces caloric intake *All amylin studies were performed in animals Pramlintide Acetate Injection US Prescribing Information, 2005 Amylin* Pramlintide

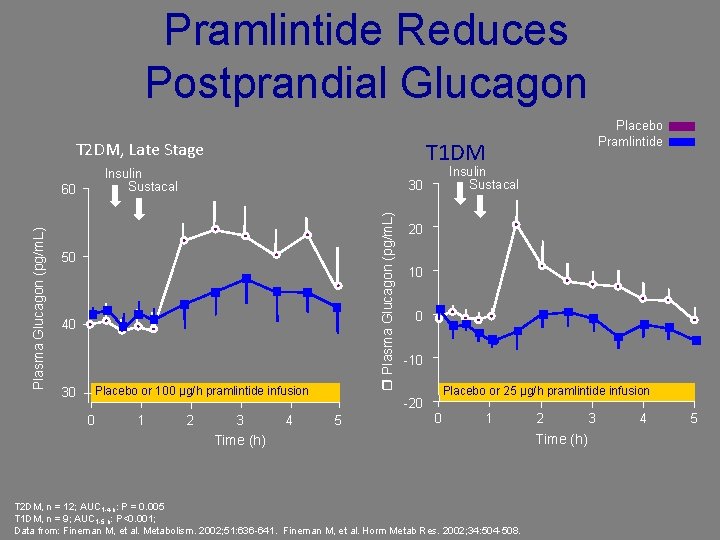
Pramlintide Reduces Postprandial Glucagon T 1 DM T 2 DM, Late Stage Insulin Sustacal 50 40 30 Placebo or 100 µg/h pramlintide infusion 0 1 Insulin Sustacal 30 Plasma Glucagon (pg/m. L) 60 2 Placebo Pramlintide 3 4 20 10 0 -10 Placebo or 25 µg/h pramlintide infusion -20 5 0 1 Time (h) T 2 DM, n = 12; AUC 1 -4 h: P = 0. 005 T 1 DM, n = 9; AUC 1 -5 h: P<0. 001; Data from: Fineman M, et al. Metabolism. 2002; 51: 636 -641. Fineman M, et al. Horm Metab Res. 2002; 34: 504 -508. 2 Time (h) 3 4 5

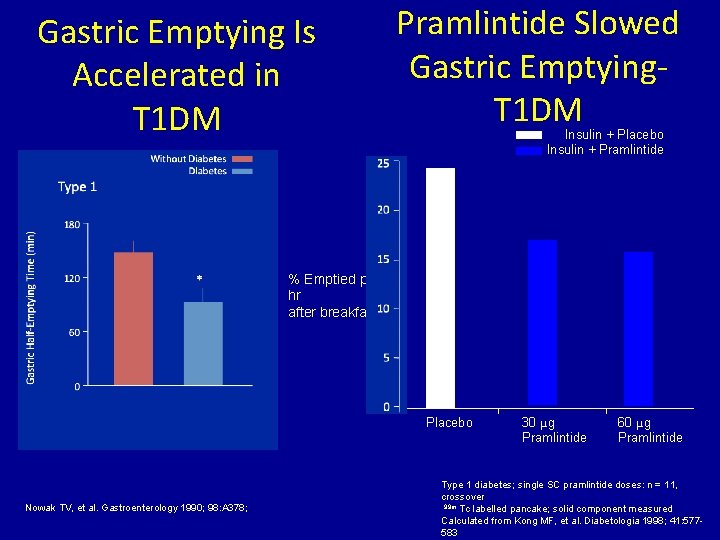
Gastric Emptying Is Accelerated in T 1 DM Pramlintide Slowed Gastric Emptying. T 1 DM Insulin + Placebo Insulin + Pramlintide % Emptied per hr after breakfast Placebo Nowak TV, et al. Gastroenterology 1990; 98: A 378; 30 μg Pramlintide 60 μg Pramlintide Type 1 diabetes; single SC pramlintide doses: n = 11, crossover 99 m Tc labelled pancake; solid component measured Calculated from Kong MF, et al. Diabetologia 1998; 41: 577583

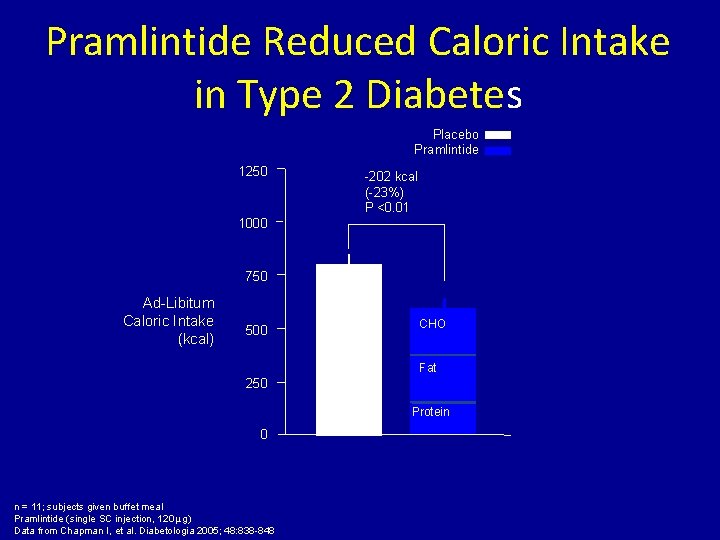
Pramlintide Reduced Caloric Intake in Type 2 Diabetes Placebo Pramlintide 1250 -202 kcal (-23%) P <0. 01 1000 750 Ad-Libitum Caloric Intake (kcal) CHO 500 Fat 250 Protein 0 n = 11; subjects given buffet meal Pramlintide (single SC injection, 120 μg) Data from Chapman I, et al. Diabetologia 2005; 48: 838 -848 Protein

Pramlintide Improved Postprandial Glucose TYPE 1 DIABETES Lispro Insulin Pramlintide 60 μg + Lispro Insulin 300 Mean (SE) Plasma Glucose (mg/d. L) 250 200 150 100 0 60 120 240 Regular Insulin Pramlintide 60 μg + Regular Insulin 300 Mean (SE) Plasma Glucose (mg/d. L) 180 250 200 150 100 0 60 120 180 240 Time Relative to Meal and Pramlintide (min) Evaluable; Mean (SE) Pramlintide + Lispro insulin, n = 20; Pramlintide + Regular insulin, n = 18 Data from Weyer C, et al. Diabetes Care 2003; 26: 3074 -3079; Pramlintide Acetate Prescribing Information, 2005

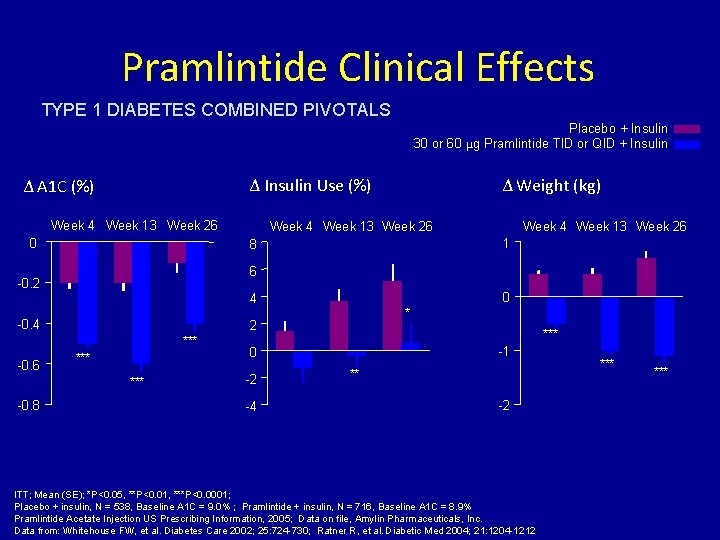
Pramlintide Clinical Effects TYPE 1 DIABETES COMBINED PIVOTALS Placebo + Insulin 30 or 60 μg Pramlintide TID or QID + Insulin Δ Insulin Use (%) Δ A 1 C (%) Week 4 Week 13 Week 26 0 Week 4 Week 13 Week 26 1 6 0 4 -0. 4 * 2 *** *** -0. 8 Week 4 Week 13 Week 26 8 -0. 2 -0. 6 Δ Weight (kg) *** -1 0 -2 -4 ** -2 ITT; Mean (SE); *P<0. 05, **P<0. 01, ***P<0. 0001; Placebo + insulin, N = 538, Baseline A 1 C = 9. 0% ; Pramlintide + insulin, N = 716, Baseline A 1 C = 8. 9% Pramlintide Acetate Injection US Prescribing Information, 2005; Data on file, Amylin Pharmaceuticals, Inc. Data from: Whitehouse FW, et al. Diabetes Care 2002; 25: 724 -730; Ratner R, et al. Diabetic Med 2004; 21: 1204 -1212 ***

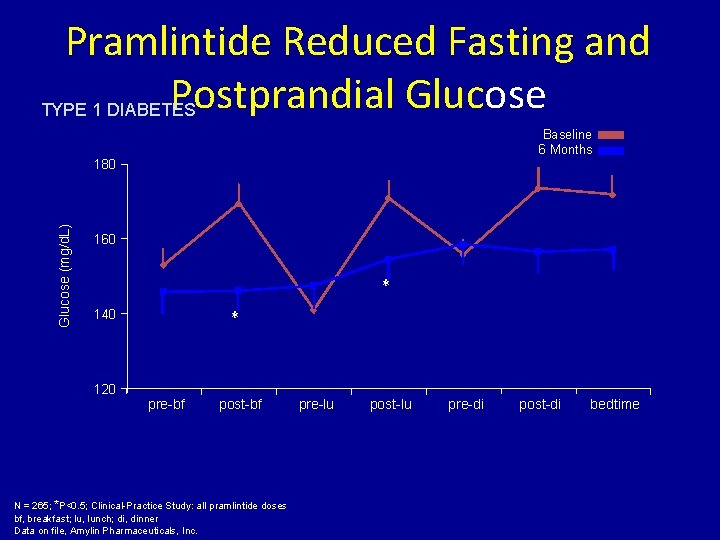
Pramlintide Reduced Fasting and Postprandial Glucose TYPE 1 DIABETES Baseline 6 Months Glucose (mg/d. L) 180 160 * * 140 120 pre-bf post-bf N = 265; *P<0. 5; Clinical-Practice Study: all pramlintide doses bf, breakfast; lu, lunch; di, dinner Data on file, Amylin Pharmaceuticals, Inc. pre-lu post-lu pre-di post-di bedtime

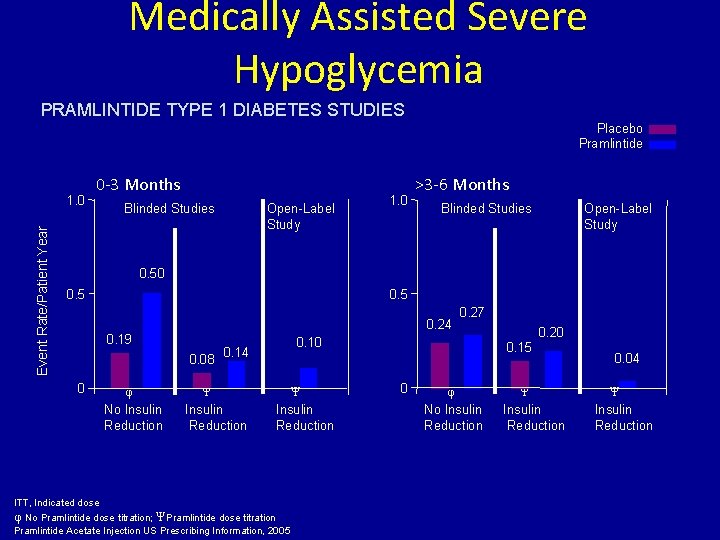
Medically Assisted Severe Hypoglycemia PRAMLINTIDE TYPE 1 DIABETES STUDIES Placebo Pramlintide Event Rate/Patient Year 1. 0 0 -3 Months Blinded Studies Open-Label Study 1. 0 >3 -6 Months Blinded Studies Open-Label Study 0. 50 0. 5 0. 24 0. 19 0. 08 0 φ No Insulin Reduction 0. 10 0. 14 Ψ Insulin Reduction 0. 27 Ψ Insulin Reduction ITT, Indicated dose φ No Pramlintide dose titration; Ψ Pramlintide dose titration Pramlintide Acetate Injection US Prescribing Information, 2005 0. 20 0. 15 0 φ No Insulin Reduction Ψ Insulin Reduction 0. 04 Ψ Insulin Reduction

Pramlintide Safety and Tolerability in Type 1 Diabetes • Insulin-Induced Severe Hypoglycemia: – More common in type 1 diabetes; risk reduced by appropriate patient selection, careful patient instruction and insulin dose adjustments as stated in the Boxed Warning • Nausea: – Mostly mild-to-moderate nausea. Occurred more frequently during initiation and then decreased with time but can increase risk of hypoglycemia. – Nausea reduced by dose titration – Could increase risk of insulin-induced severe hypoglycemia due to reduced food intake Pramlintide Acetate Injection US Prescribing Information, 2005
 B-pleated sheet
B-pleated sheet Tôn thất thuyết là ai
Tôn thất thuyết là ai Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart Gây tê cơ vuông thắt lưng
Gây tê cơ vuông thắt lưng Block nhĩ thất độ 2 mobitz 1
Block nhĩ thất độ 2 mobitz 1 Tìm độ lớn thật của tam giác abc
Tìm độ lớn thật của tam giác abc Sau thất bại ở hồ điển triệt
Sau thất bại ở hồ điển triệt Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Hãy nói thật ít để làm được nhiều
Hãy nói thật ít để làm được nhiều Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật



















