Post it Pollutants are dangerous chemicals in the













- Slides: 25

Post it Pollutants are dangerous chemicals in the air are called pollutants. Name as many air pollutants as you can from last lesson. Extension: Circle those which could lead to acid rain

Hydrocarbons and fossil fuels To develop an understanding of how air pollutants are produced

Grade criteria Grade A* - Generate combustion reactions using balance symbol equations. Grade A – Summarise combustion reactions using word equations. Grade B – Explain how atoms are conserved during combustion reactions. Grade C – Identify hydrocarbons and fossil fuels by their molecular pictures Grade D –Define the terms ‘hydrocarbon’ and ‘fossil fuels’, giving examples of each

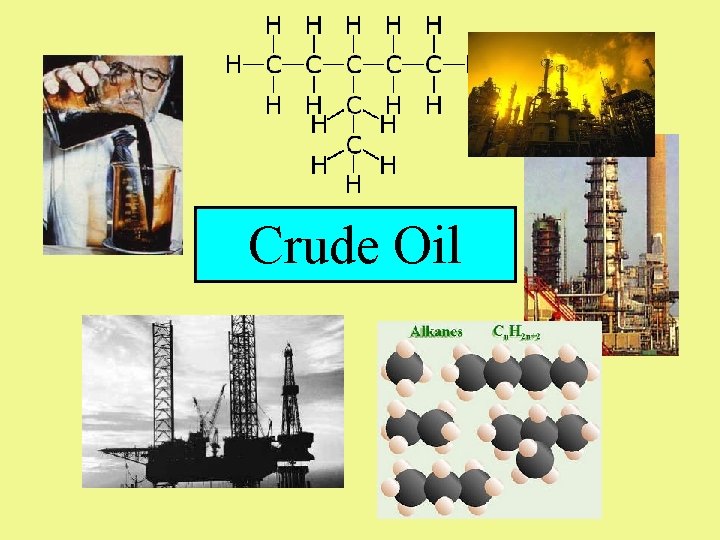
Combustion, fuels and hydrocarbons When a substance burns, it is said to combust. Combustion is a rapid reaction between a substance and oxygen that releases heat and light energy. A fuel is a substance that reacts with oxygen (combusts) to release useful energy. Many fractions obtained from crude oil are used as fuels because they contain hydrocarbons that burn easily and release a large amount of useful energy.

Hydrocarbons – What’s so good about them? • • Contain carbon and hydrogen atoms only Can have different carbon chain lengths Length of the chain determines the use Crude oil is a mixture of lots and lots of different hydrocarbons.

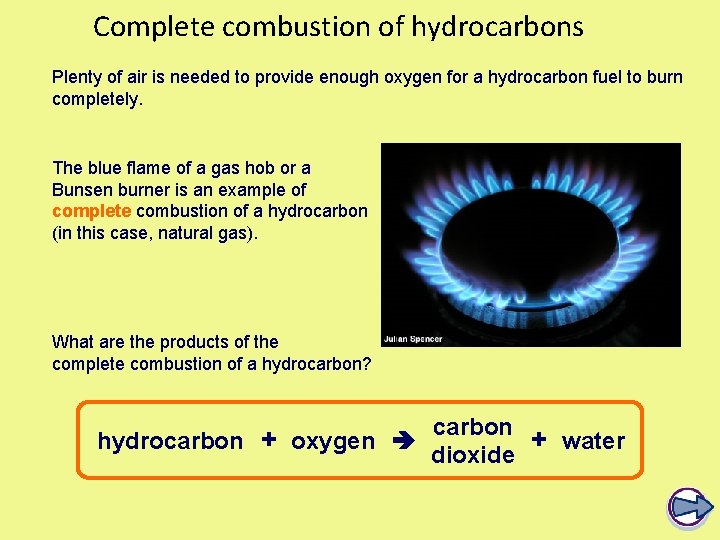
Complete combustion of hydrocarbons Plenty of air is needed to provide enough oxygen for a hydrocarbon fuel to burn completely. The blue flame of a gas hob or a Bunsen burner is an example of complete combustion of a hydrocarbon (in this case, natural gas). What are the products of the complete combustion of a hydrocarbon? carbon hydrocarbon + oxygen + water dioxide


Do you think that combustion is as simple as: carbon hydrocarbon + oxygen + water dioxide

VCOP – What are particulates? Consider your observations, which flame would it have been?

Grade criteria Grade A* - Generate combustion reactions using balance symbol equations. Grade A – Summarise combustion reactions using word equations. Grade B – Explain how atoms are conserved during combustion reactions. Grade C – Identify hydrocarbons and fossil fuels by their molecular pictures Grade D –Define the terms ‘hydrocarbon’ and ‘fossil fuels’, giving examples of each

Plenary: Methane bubbles • What is a hydrocarbon? Name an example • Give a general word equation for combustion • Name a product of incomplete combustion? How is it harmful? • Balance the equation

Homework • Complete pages 20 – 23/24/25

For each answer below write as many questions as you can think of I am the answer. . Hydrocarbon Acid rain Nitrogen Monoxide Combustion Carbon Dioxide + Water Crude oil So I am the Question

Explain why crude oil is an invaluable resource • Know the origins of crude oil • Describe the process to separate crude oil • Recognise the uses of the fractions of crude oil

What genre of chemicals do you get?

Hydrocarbons – What’s so good about them? • • Contain carbon and hydrogen atoms only Can have different carbon chain lengths Length of the chain determines the use Crude oil is a mixture of lots and lots of different hydrocarbons.

Crude Oil

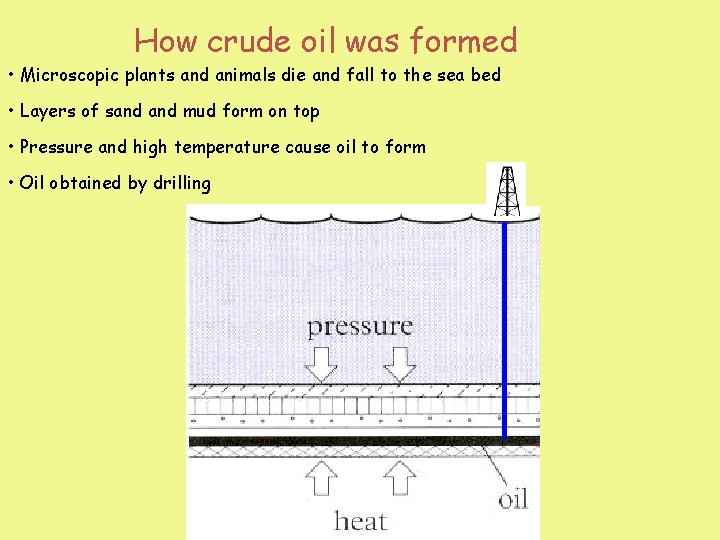
How crude oil was formed • Microscopic plants and animals die and fall to the sea bed • Layers of sand mud form on top • Pressure and high temperature cause oil to form • Oil obtained by drilling

Explain why crude oil is an invaluable resource • Know the origins of crude oil • Describe the process to separate crude oil • Recognise the uses of the fractions of crude oil

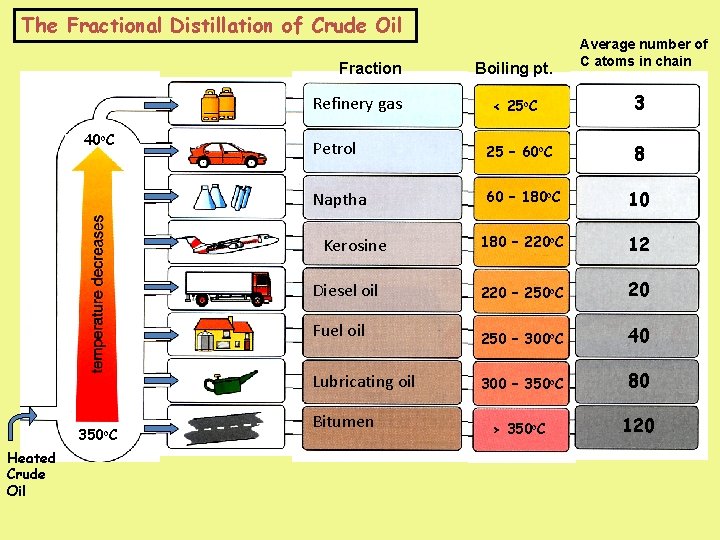
The Fractional Distillation of Crude Oil Fraction Refinery gas 40 o. C 3 25 – 60 o. C Naptha 60 – 180 o. C 10 180 – 220 o. C 12 220 – 250 o. C 20 250 – 300 o. C 40 300 – 350 o. C 80 > 350 o. C 120 Diesel oil Fuel oil Lubricating oil Heated Crude Oil < 25 o. C Petrol Kerosine 350 o. C Boiling pt. Average number of C atoms in chain Bitumen 8

Explain why crude oil is an invaluable resource • Know the origins of crude oil • Describe the process to separate crude oil • Recognise the uses of the fractions of crude oil

The Molymod challenge • Rules Carbon atoms always make 4 bonds • Hydrogen atoms are smaller • Hydrogen atoms can only make one bond Make the molecule methane (1 Carbon to 4 Hydrogens) Make the molecule propane (3 carbons to ? Hydrogens) Make the molecule hexane (? Carbons to 14 Hydrogens)

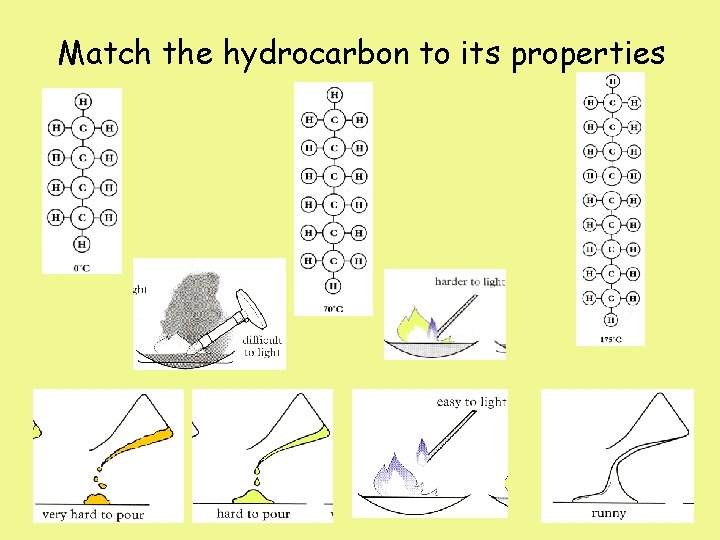
Match the hydrocarbon to its properties

Match the hydrocarbon to its properties

carbon monoxide dioxide sulfur dioxide formula CO SO 2 carbon atoms 1 nitrogen monoxide dioxide sulfur atoms nitrogen atoms hydrogen atoms oxygen atoms 1 Extension: what is the word equation for combustion? water (hydrogen oxide)
 Planting more trees
Planting more trees Primary pollutants and secondary pollutants
Primary pollutants and secondary pollutants Primary and secondary air pollutants
Primary and secondary air pollutants Mikael ferm
Mikael ferm Commodity vs specialty chemicals
Commodity vs specialty chemicals Inorganic gases
Inorganic gases What is secondary pollutant
What is secondary pollutant Major air pollutants
Major air pollutants What is secondary pollutant
What is secondary pollutant Solar energy and the atmosphere
Solar energy and the atmosphere What are the secondary air pollutants
What are the secondary air pollutants Primary and secondary pollutants difference
Primary and secondary pollutants difference Primary vs secondary pollution
Primary vs secondary pollution Air pollutants
Air pollutants Primary vs secondary pollutants
Primary vs secondary pollutants Is environmental pollution
Is environmental pollution Differentiate between primary and secondary pollutants
Differentiate between primary and secondary pollutants Short lived climate pollutants
Short lived climate pollutants Stock pollutants
Stock pollutants Secondary pollutants examples
Secondary pollutants examples Environmental pollution meaning
Environmental pollution meaning Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Indoor air pollution sources
Indoor air pollution sources Aaditya chemicals
Aaditya chemicals Boston chemicals
Boston chemicals Shriji chemicals
Shriji chemicals