Plasma Derivatives Cleaning Procedures and Clearance of TSE














- Slides: 19

Plasma Derivatives: Cleaning Procedures and Clearance of TSE Agents During Manufacturing TSE Advisory Committee July 18, 2003 Dorothy Scott, M. D. Laboratory of Plasma Derivatives/OBRR/CBER

Use of Common Equipment for Manufacturing of U. S. and European Plasma for Plasma Derivatives in the Context of v. CJD Risk • Many manufacturers use common equipment for U. S. and European plasma – Approved to do so as part of license or licensing supplements – Products include: IGIV, albumin, FVIII, FIX – At least 5 major manufacturers of plasma derivatives are licensed to use common equipment and facilities for U. S. and European plasma • v. CJD donor deferrals differ between U. S. and European countries

Recommended Plasma Donor Deferrals in U. S. and Europe for v. CJD risk Deferral time for residence U. K. France Europe U. S. : SP 1 > 3 mos. > 5 years no deferral U. S. : RP 2 > 3 mos. > 5 years Europe 3 0 – 5 yrs. 0 – 6 mos no deferral Europe EMEA 4 1 year none Source Plasma; 2 Recovered Plasma; 3 Plasma; 4 EMEA CPMP Position Statement on CJD and plasma-derived and urine-derived medicinal products 2/2003 (http: //www. emea. eu. int/htms/human/tse. htm). 1

Considerations in the Context of Risks of TSE Transmission via Reused Equipment and Materials • Amount of agent in starting material (plasma) – Presence and titer in plasma – Deferral of at-risk donors • Clearance (or concentration) by manufacturing processes • Evaluation of the cleaning procedure for potential chemical inactivation of TSE agents should consider these influences on overall risk

Reasons to Consider Decontamination of Facilities and Equipment for Plasma Derivatives • Possibility that cross-contamination could occur; donor may be diagnosed too late to interdict plasma use • Unpredictability of human TSE outbreaks – possibilities for the future – More BSE countries continue to be identified (e. g. Canada) – In theory, prion codon 129 heterozygotes could develop v. CJD (this would increase epidemic size) – Chronic wasting disease in U. S. – spread to humans or domestic animals not impossible – Analogous and connected to importance of continuing food chain controls

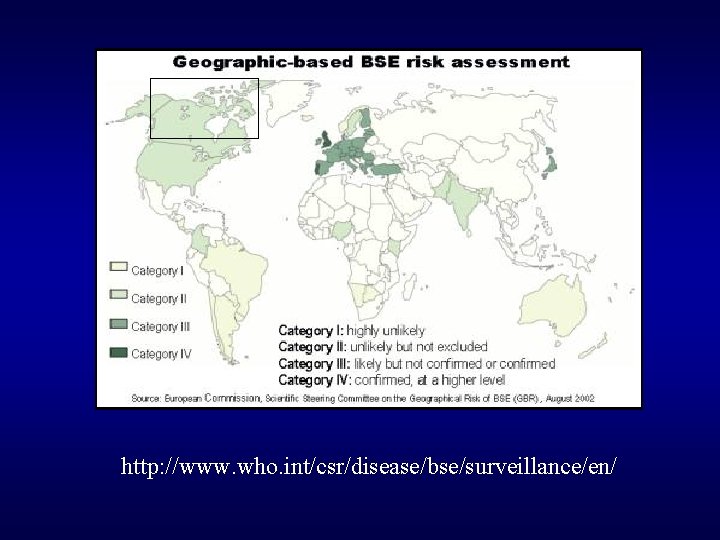
http: //www. who. int/csr/disease/bse/surveillance/en/

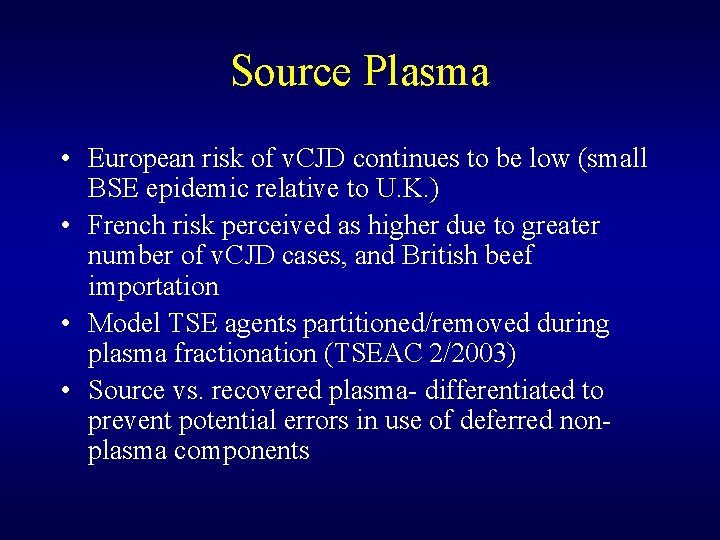
Source Plasma • European risk of v. CJD continues to be low (small BSE epidemic relative to U. K. ) • French risk perceived as higher due to greater number of v. CJD cases, and British beef importation • Model TSE agents partitioned/removed during plasma fractionation (TSEAC 2/2003) • Source vs. recovered plasma- differentiated to prevent potential errors in use of deferred nonplasma components

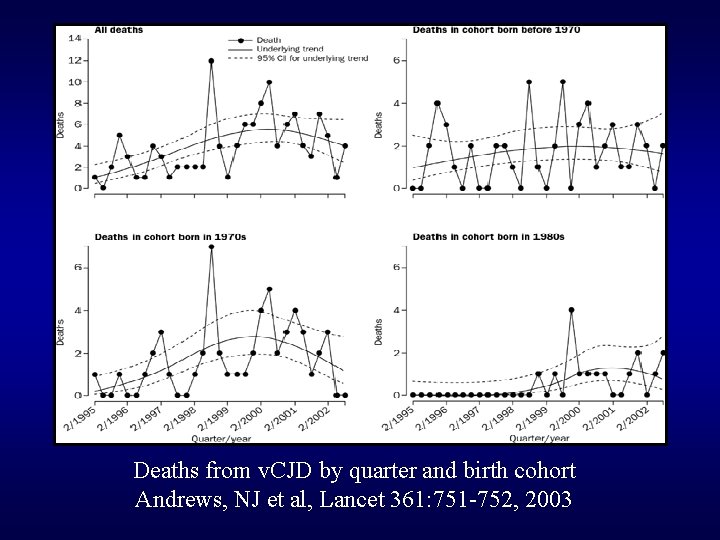
Deaths from v. CJD by quarter and birth cohort Andrews, NJ et al, Lancet 361: 751 -752, 2003

v. CJD Cases in Europe • • France (6) Italy (1) Ireland (1) (Czech Republic? ) (1) Post-donation diagnosis of v. CJD in a European donor is possible where plasma derivatives will have been processed

TSE infectivity clearance during plasma derivative manufacturing • Manufacturing steps that can result in TSE clearance – Precipitations – Depth filtration – Column Chromatography • Clearance is process and manufacturer-specific (because it is context-specific) • Manufacturers invited to submit clearance data to FDA for consideration of labeling claims (TSEAC 2/20/2003 at http: //www. fda. gov/ohrms/dockets/ac/03/transcrip ts/3923 t 1. htm

Cleaning Procedures Between Plasma Batches (Campaigns) • Validation of equipment cleaning procedures is standard for licensure (but validation not TSEspecific) • Examples of cleaning methods – Na. OH and/or Na. OCl solutions – Rinsing • Examples of cleaning validation test methods – Residual total organic carbon – Residual protein – Ionic strength of final rinsing solutions

Equipment and Process Materials in Plasma Derivative Manufacturing • Usually reused after cleaning: – – – – Plasma pooling equipment Stainless steel tanks Tangential flow filters* Gaskets and tubing Sterile filtration/final filling machinery Affinity chromatography columns* Some resins* • Usually Disposable: – Sterile filters – Depth filters – Some resins (e. g. anion, cation exchange columns) * Dedicated to U. S. -source plasma

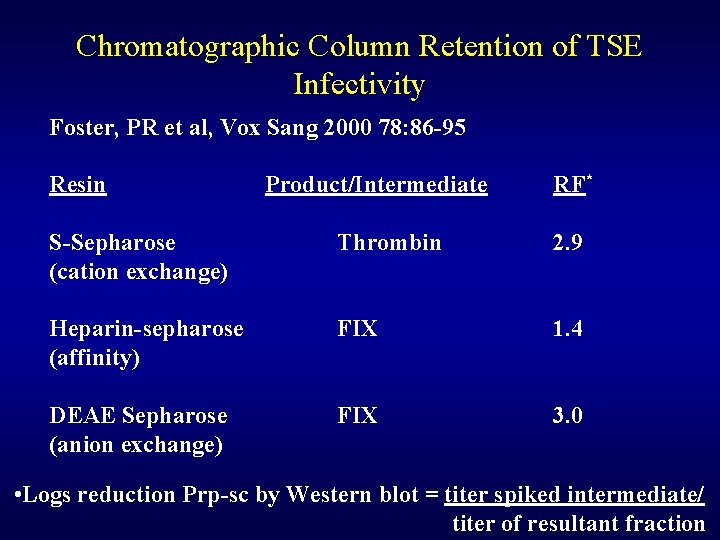
Chromatographic Column Retention of TSE Infectivity Foster, PR et al, Vox Sang 2000 78: 86 -95 Resin Product/Intermediate RF* S-Sepharose (cation exchange) Thrombin 2. 9 Heparin-sepharose (affinity) FIX 1. 4 DEAE Sepharose (anion exchange) FIX 3. 0 • Logs reduction Prp-sc by Western blot = titer spiked intermediate/ titer of resultant fraction

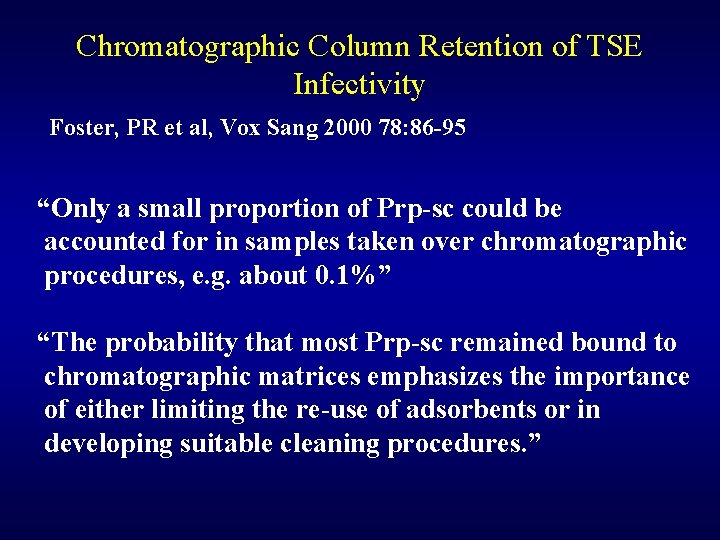
Chromatographic Column Retention of TSE Infectivity Foster, PR et al, Vox Sang 2000 78: 86 -95 “Only a small proportion of Prp-sc could be accounted for in samples taken over chromatographic procedures, e. g. about 0. 1%” “The probability that most Prp-sc remained bound to chromatographic matrices emphasizes the importance of either limiting the re-use of adsorbents or in developing suitable cleaning procedures. ”

TSE Adherence to Chromatographic Resins • Demonstrated in other published studies: 102 – 105 fold reduction in scrapie infectivity – Hunter and Millson (J. Gen. Microb. 37: 251 -8) – Kozak et al (Dev. Biol. Stand. 88: 257 -64, 1996) – Blum et al (Biopharm. 11: 28 -34, 1998) • Reported for anion exchange, cation exchange, hydrophobic interaction, and affinity chromatography • Some chromatographic resins (e. g. some anion, cation) may be washed with 0. 1 -1 M Na. OH; others (e. g. antibody affinity columns and hydrophobic interaction columns) are not chemically stable under alkaline conditions

Considerations in the Context of Risks of TSE Transmission via Reused Equipment and Materials • Amount of agent in starting material (plasma) – Presence and titer in plasma believed to be low, if present – Deferral of at-risk donors from the U. K. , in particular, limits the number of possible incubating donors • Clearance by manufacturing processes – Demonstrated in general for many common procedures used in plasma derivative manufacturing; submission of rigorous and process specific studies to FDA requested on a voluntary basis • Evaluation of cleaning for TSE agents should consider these influences on overall risk

Questions to the Committee: Plasma Derivatives 1. Are current facility cleaning methods, e. g. the use of solutions of sodium hydroxide or sodium hypochlorite followed by extensive rinsing cycles, adequate to minimize the possibility that an infectious dose of the v. CJD agent may be carried over from one manufactured lot into the next?

Questions to the Committee: Plasma Derivatives 2. Are the available scientific data sufficient for FDA to recommend specific methods for cleaning of equipment used in the manufacture of plasma derivatives with respect to TSE clearance or inactivation? a. If so, please identify which methods can be recommended b. If not, please describe what additional studies would assist in development of such recommendations

Speakers: Methods to Decontaminate Facilities and Equipment for Plasma Derivatives, to Reduce Possible Risk of TSE Transmission • Decontamination Practices for Plasma Product Facilities – Dr. Cristoph Kempf, PPTA (Plasma Protein Therapeutics Association) • Proposed PPTA-sponsored Collaborative Studyon Inactivation of TSE Agents with Sodium Hydroxide and Sodium Hypochlorite, Commonly Used to Clean and Decontaminate Facilities and Equipment in Manufacture of Plasma Derivatives – Dr. Andrew Bailey, PPTA
 Febra w pustyni i w puszczy
Febra w pustyni i w puszczy Hospital patient room cleaning procedures
Hospital patient room cleaning procedures Operating room cleaning procedures
Operating room cleaning procedures Hospital patient room cleaning procedures
Hospital patient room cleaning procedures Tse/bse meaning
Tse/bse meaning K-q tse-iso-en 9000 logo
K-q tse-iso-en 9000 logo Nasyonalismo sa silangan
Nasyonalismo sa silangan Elizabeth tse
Elizabeth tse Dr michael tse
Dr michael tse Fundamentals of wireless communication solution
Fundamentals of wireless communication solution Tse-yu yeh
Tse-yu yeh Mao tse-tung
Mao tse-tung K-q tse-iso-en 9000
K-q tse-iso-en 9000 Pmh questions
Pmh questions Cruos
Cruos Big data
Big data Clifford tse
Clifford tse Julie tse
Julie tse Blackberry stock after hours
Blackberry stock after hours Tse group
Tse group