TSE Clearance in Plasma Derivatives TSE Advisory Committee











- Slides: 16

TSE Clearance in Plasma Derivatives TSE Advisory Committee February 8, 2005 Dorothy Scott, M. D. DH/OBRR/CBER/FDA

TSE Clearance Studies and Risk Assessment • Clearance is an important factor in overall risk estimation • Clearance by manufacturing process CAN be tested in scaled-down studies • Viral clearance studies paradigm applied

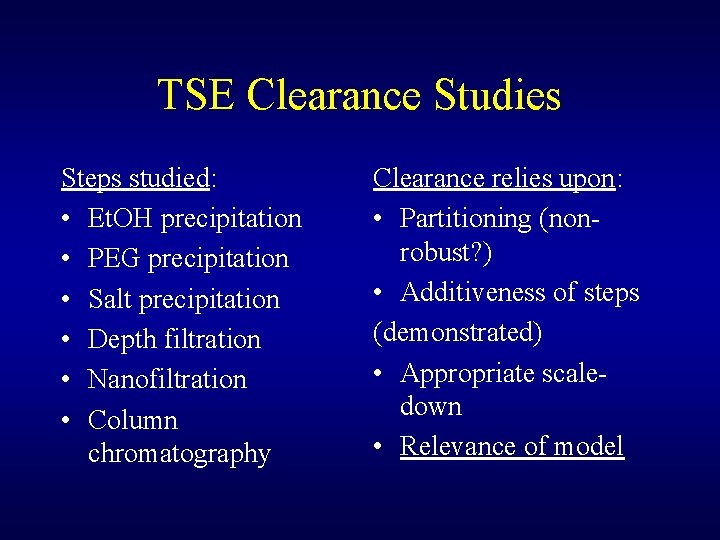
Paradigm: Validation of Virus Removal/inactivation Includes: • Scaling down process steps • Spiking appropriate steps with high titer of infectious agent (actual or model) • Determination reduction factors for each step • Summing reduction factors [from nonorthogonal processes] to give a total log 10 reduction value

Studies of Clearance of TSE Agents • Source of infectivity – Brain preparations from experimentally infected animals with human/animal TSE agents – Blood from experimentally infected animals • Form infectious agent – – – Brain homogenate Subcellular fractions Membrane-free infectious material (e. g. fibrils) Blood and blood fractions * Alterations in form during manufacturing (“conditioning”)

Measures of Clearance • Assays to measure outcomes – In vivo infectivity – laborious, expensive, longterm experiments, but considered most relevant and most sensitive – In vitro - measurements of Pr. PSc – Bridging in vivo to in vitro results scientific controversy exists

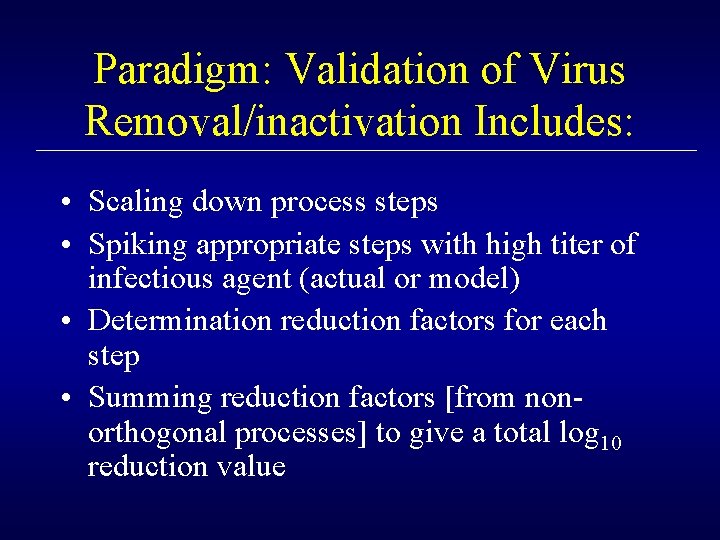
TSE Clearance Evaluation: Spiking Model TSE Spike Plasma Cryoprecipitation Cryoprecipitate (FVIII) Cryopoor Plasma Supernatant Albumin, IGIV, A 1 PI, etc.

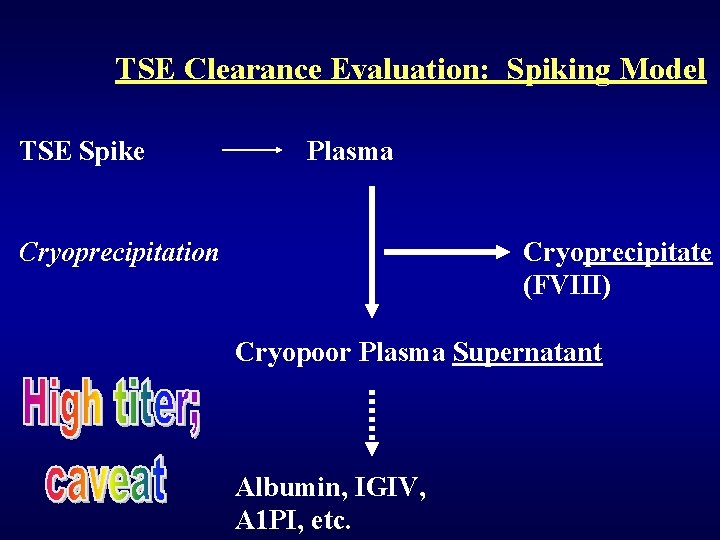
TSE Clearance Evaluation: Endogenous Infection model Plasma from TSE-infected animal Cryoprecipitation Cryoprecipitate (FVIII) Cryopoor Plasma Supernatant Albumin, IGIV, A 1 PI, etc.

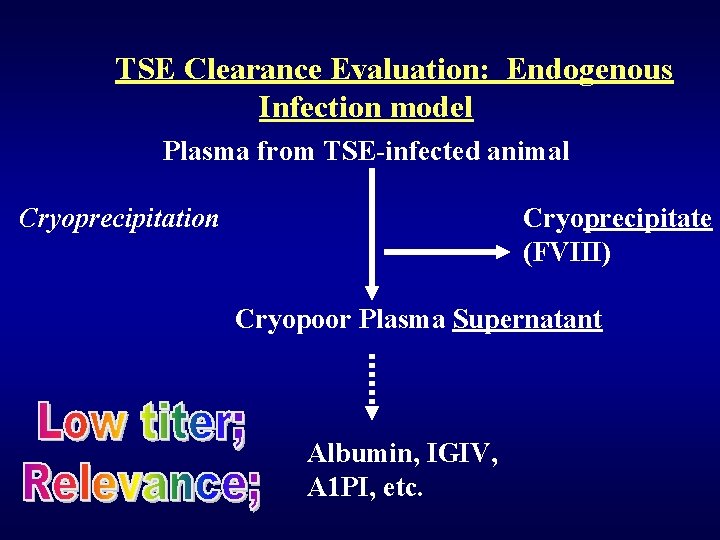
TSE Clearance Studies Steps studied: • Et. OH precipitation • PEG precipitation • Salt precipitation • Depth filtration • Nanofiltration • Column chromatography Clearance relies upon: • Partitioning (nonrobust? ) • Additiveness of steps (demonstrated) • Appropriate scaledown • Relevance of model

TSE Clearance and Individual Manufacturing Processes • Manufacturing processes are highly individual • Rigorous demonstrations of TSE clearance need to be based upon the specific manufacturing process

Specificity of Process: Clearance Pr. Psc (microsomal spike) by Depth Filtration – Influence of Starting Materials and Filter Starting Material Depth Filter Reduction Factor (log 10) Fr V (albumin) Seitz KS 80 > 4. 9 Fr V (albumin) CUNO Delipid 1 2. 3 S I + III (IGIV) Millipore AP 20 <1 Fr II (IGIV) Seitz K 200 > 2. 8 Foster et. al. , Vox Sang 78: 86 -95, 2000 Fr I supernatant (IGIV, albumin) Supra P 80 <1 Fr V supernatant (albumin) Supra P 80 > 1. 1 Fr V supernatant (albumin) – Prp-sc spike Supra P 80 > 2. 4 Vey et al, Biologicals 30: 187 -96, 2002

OBRR Actions to Minimize Risk of TSE Agents in Blood Products – TSE Clearance TSEAC (2/2003) endorsed FDA consideration of labeling claims for TSE clearance in plasma derivatives, based upon specific demonstration of TSE removal during manufacturing • TSE clearance study submissions encouraged by OBRR – Submissions received, evaluations in progress

FDA Requests for Submission TSE Clearance Data • Voluntary • Best current methods • Model selection not restricted but needs to be justified • 3 Logs clearance for “non-robust” steps considered significant • Science-in-evolution

TSE Clearance and Risk Assessment • TSE clearance a critical variable in risk assessments for v. CJD • Clearance can be tested on a laboratory scale, with caveats (spike relevance, model agents, etc. ) • Data can be provided for risk assessments: specific study of product provides best approximation of clearance • Clearance studies, and advances in these study methods could improve precision of risk estimates

Published TSE Clearance Studies for Plasma Derivatives (1) 1. Brown, P et al. The distribution of infectivity in blood components and plasma derivatives in experimental models of transmissible spongiform encephalopathy. Transfusion 1998 38: 810 -6 2. Brown, P et al. Further studies of blood infectivity in an experimental model of transmissible spongiform encephalopathy, with an explanation of why blood components do not transmit CJD in humans. Transfusion 1999 39: 1169 -78 3. Lee, DC et al. Monitoring plasma processing steps with a sensitive Western blot assay for the detection of prion protein. J. Virol. Meth. 2000 84: 77 -89 4. Foster, PR et al. Assessment of the potential of plasma fractionation processes to remove causative agents of transmissible spongiform encephalopathy. Transfusion Science 2000 22: 53 -56 5. Foster, PR et al. Assessment of the potential of plasma fractionation processes to remove causative agents of transmissible spongiform encephalopathy. Vox Sanguinis 2000 78: 86 -95 6. Lee, DC et al. A direct relationship between the partitioning of the pathogenic prion protein and transmissible spongiform encephalopathy infectivity during the purification of plasma proteins. Transfusion 2001 41: 449 -55

Published TSE Clearance Studies for Plasma Derivatives (2) 7. Cai, K et al. Solvent-dependent precipitation of prion protein. Biochem Biophys. Acta 2002 1597: 28 -35 8. Stenland, JS et al. Partitioning of human and sheep forms of the pathogenic prion protein during the purification of therapeutic proteins from human plasma. Transfusion 2002 42: 1497 -1500 9. Vey, M et al. Purity of spiking agent affects partitioning of prions in plasma protein purification. Biologicals 2002 30: 187 -96 10. Reichl, HE et al. Studies on the removal of a BSE-derived agent by processes used in the manufacture of human immunoglobulin. Vox Sanguinis 2002 83: 137 -45 11. Van Holten, RW et al. Removal of prion challenge from an immune globulin preparation by use of a size-exclusion filter. Transfusion 2002 42: 973 -4. 12. Van Holten RW et al. Evaluation of depth filtration to remove prion challenge from an immune globulin preparation. Vox Sang 2003 85: 20 -4.

Published TSE Clearance Studies for Plasma Derivatives (3) 13. Trejo, SR, et al. Evaluation of virus and prion reduction in a new intravenous immunoglobulin manufacturing process. Vox Sang 2003 84: 176 -87. 14. Burnouf T et al. Nanofiltration of single plasma donations: feasibility study. Vox Sang 2003 84: 111 -119. 15. Gregori, et al. Partitioning of TSE infectivity during ethanol fractionation of human plasma. Biologicals 2004 32: 1 -10. 16. Foster, PR et al. Distribution of a bovine spongiform encephalopathy-derived agen over ion-exchange chromatography used in the preparation of concentrates of fibrinogen and factor VIII. Vox Sang 2004 86: 92 -9.
 Choroby w pustyni iw puszczy
Choroby w pustyni iw puszczy Trade union advisory committee
Trade union advisory committee Robert kerzner
Robert kerzner Nasa astrophysics advisory committee
Nasa astrophysics advisory committee Aviation rulemaking advisory committee
Aviation rulemaking advisory committee Learning without burden was published in
Learning without burden was published in Isda determinations committee
Isda determinations committee Elizabeth tse
Elizabeth tse Atomic leow
Atomic leow Iso 9000-1
Iso 9000-1 Chiang kai shek
Chiang kai shek Tse bse certificate in pharmaceutical
Tse bse certificate in pharmaceutical Va form 21p-0969 income and asset statement
Va form 21p-0969 income and asset statement Dr michael tse
Dr michael tse Dr tse lung fung
Dr tse lung fung K-q tse-iso-en 9000 logo
K-q tse-iso-en 9000 logo Julie tse
Julie tse































