PlanarChiral Macrocyclic Host Pillar5arene No Rotation of Units
![Planar-Chiral Macrocyclic Host Pillar[5]arene: No Rotation of Units and Isolation of Enantiomers by Introducing Planar-Chiral Macrocyclic Host Pillar[5]arene: No Rotation of Units and Isolation of Enantiomers by Introducing](https://slidetodoc.com/presentation_image_h2/0e3e7505b77517f26799c7b5a5e54729/image-1.jpg)
![Pillar[5]arene cyclodextrin 2/25/2011 The composition of pillar[5]arene is almost the same as that of Pillar[5]arene cyclodextrin 2/25/2011 The composition of pillar[5]arene is almost the same as that of](https://slidetodoc.com/presentation_image_h2/0e3e7505b77517f26799c7b5a5e54729/image-2.jpg)
- Slides: 10
![PlanarChiral Macrocyclic Host Pillar5arene No Rotation of Units and Isolation of Enantiomers by Introducing Planar-Chiral Macrocyclic Host Pillar[5]arene: No Rotation of Units and Isolation of Enantiomers by Introducing](https://slidetodoc.com/presentation_image_h2/0e3e7505b77517f26799c7b5a5e54729/image-1.jpg)
Planar-Chiral Macrocyclic Host Pillar[5]arene: No Rotation of Units and Isolation of Enantiomers by Introducing Bulky Substituents Tomoki Ogoshi, * Kae Masaki, Ryohei Shiga, Keisuke Kitajima, and Tada-aki Yamagishi Graduate School of Natural Science and Technology, Kanazawa University, Kakuma-machi, Kanazawa 920 -1192, Japan 2/25/2011 1 Received January 10, 2011
![Pillar5arene cyclodextrin 2252011 The composition of pillar5arene is almost the same as that of Pillar[5]arene cyclodextrin 2/25/2011 The composition of pillar[5]arene is almost the same as that of](https://slidetodoc.com/presentation_image_h2/0e3e7505b77517f26799c7b5a5e54729/image-2.jpg)
Pillar[5]arene cyclodextrin 2/25/2011 The composition of pillar[5]arene is almost the same as that of typical calixarenes. 2

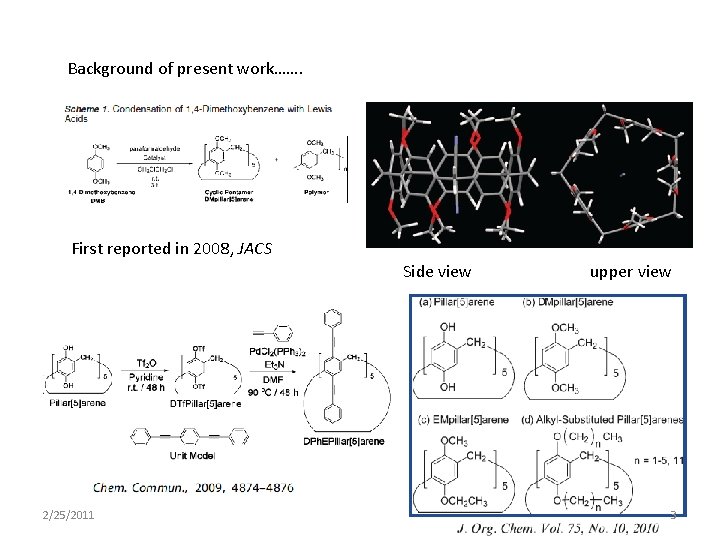
Background of present work……. First reported in 2008, JACS Side view 2/25/2011 upper view 3

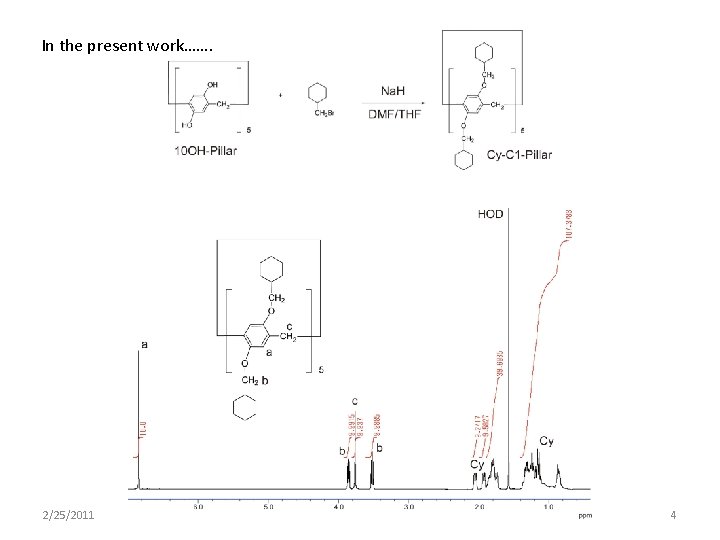
In the present work……. 2/25/2011 4

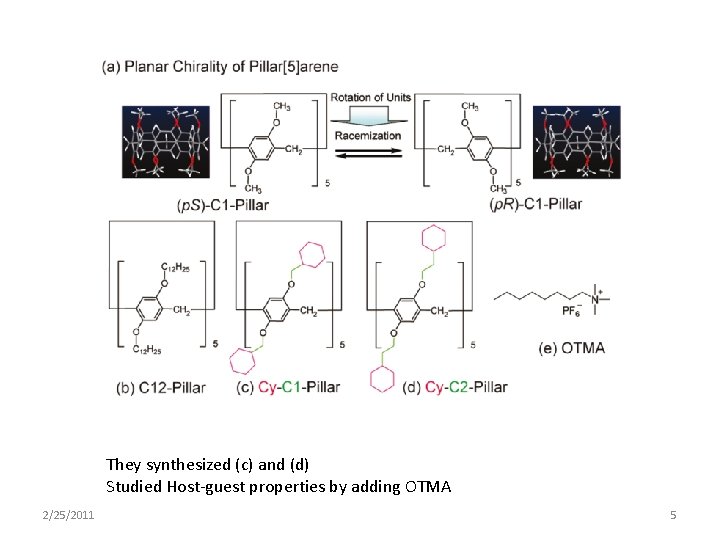
They synthesized (c) and (d) Studied Host-guest properties by adding OTMA 2/25/2011 5

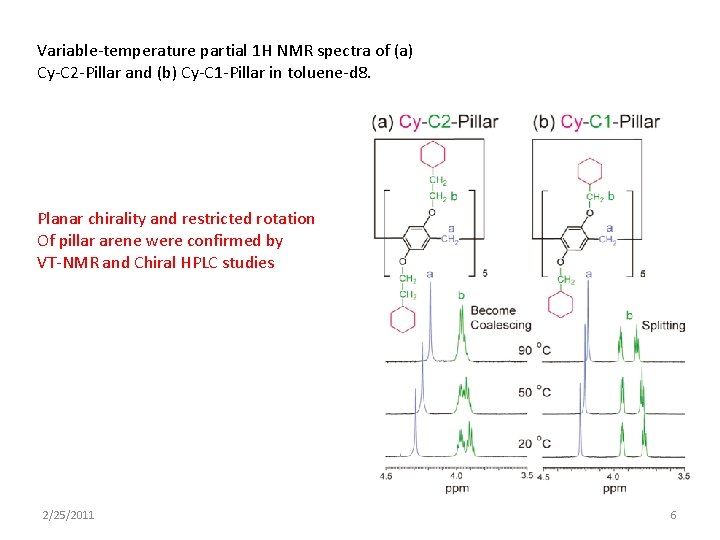
Variable-temperature partial 1 H NMR spectra of (a) Cy-C 2 -Pillar and (b) Cy-C 1 -Pillar in toluene-d 8. Planar chirality and restricted rotation Of pillar arene were confirmed by VT-NMR and Chiral HPLC studies 2/25/2011 6

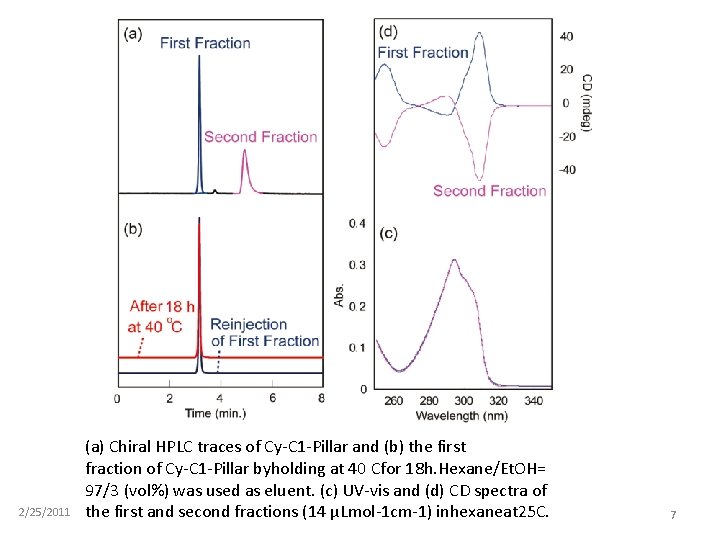
2/25/2011 (a) Chiral HPLC traces of Cy-C 1 -Pillar and (b) the first fraction of Cy-C 1 -Pillar byholding at 40 Cfor 18 h. Hexane/Et. OH= 97/3 (vol%) was used as eluent. (c) UV-vis and (d) CD spectra of the first and second fractions (14 μLmol-1 cm-1) inhexaneat 25 C. 7

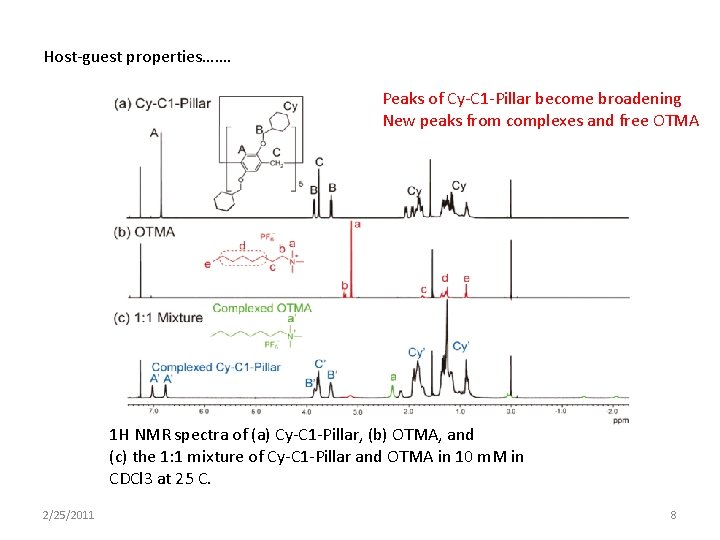
Host-guest properties……. Peaks of Cy-C 1 -Pillar become broadening New peaks from complexes and free OTMA 1 H NMR spectra of (a) Cy-C 1 -Pillar, (b) OTMA, and (c) the 1: 1 mixture of Cy-C 1 -Pillar and OTMA in 10 m. M in CDCl 3 at 25 C. 2/25/2011 8

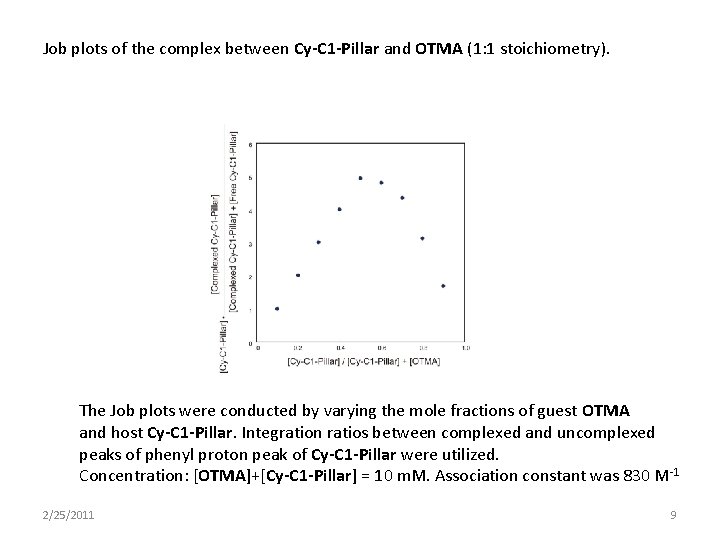
Job plots of the complex between Cy-C 1 -Pillar and OTMA (1: 1 stoichiometry). The Job plots were conducted by varying the mole fractions of guest OTMA and host Cy-C 1 -Pillar. Integration ratios between complexed and uncomplexed peaks of phenyl proton peak of Cy-C 1 -Pillar were utilized. Concentration: [OTMA]+[Cy-C 1 -Pillar] = 10 m. M. Association constant was 830 M-1 2/25/2011 9

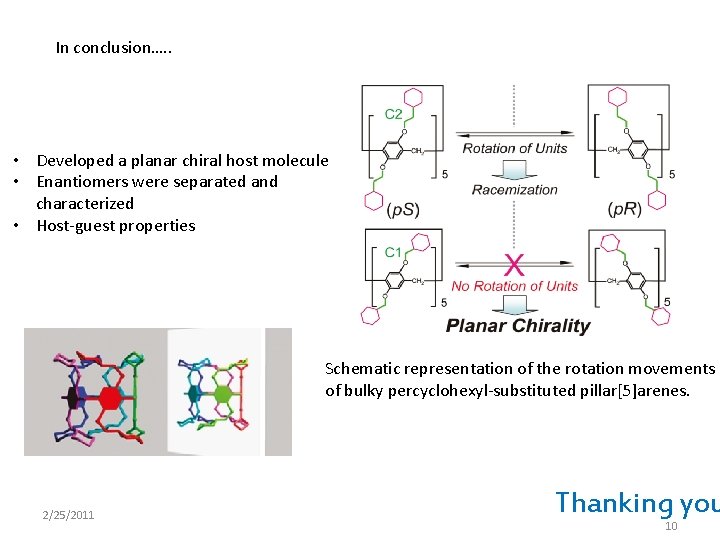
In conclusion…. . • Developed a planar chiral host molecule • Enantiomers were separated and characterized • Host-guest properties Schematic representation of the rotation movements of bulky percyclohexyl-substituted pillar[5]arenes. 2/25/2011 Thanking you 10
 Definitive host vs intermediate host
Definitive host vs intermediate host Specific rotation of sugar solution
Specific rotation of sugar solution Specific rotation units
Specific rotation units Specific optical rotation formula
Specific optical rotation formula Fischer projection to tetrahedral
Fischer projection to tetrahedral When units manufactured exceed units sold:
When units manufactured exceed units sold: Xeomin reconstitution chart
Xeomin reconstitution chart Types of intelligent storage system
Types of intelligent storage system Kamyar niroumand
Kamyar niroumand Host hardening
Host hardening Umdf driver
Umdf driver