Macrolides consist of macrocyclic lactone 12 14 16


- Slides: 11

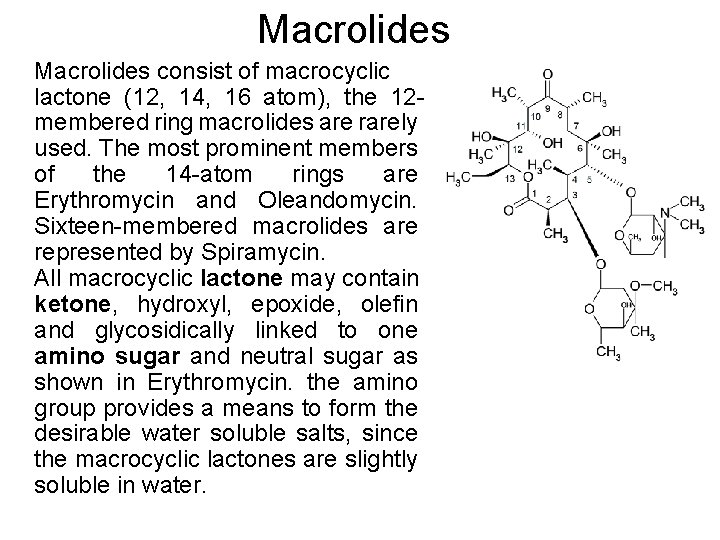
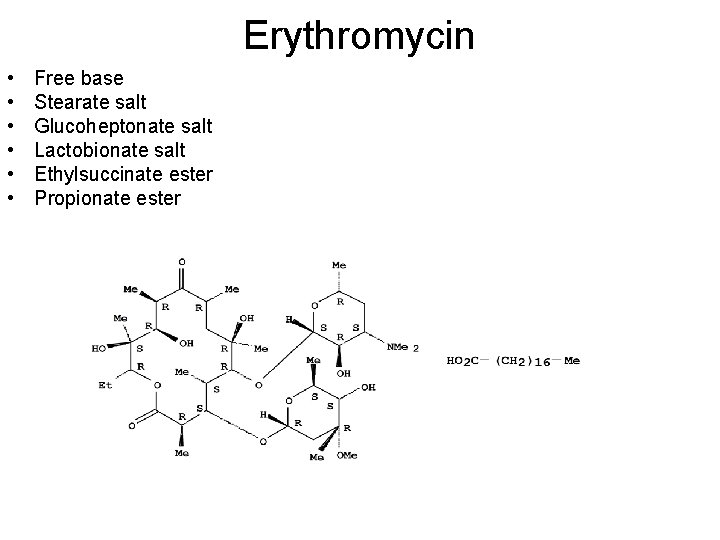
Macrolides consist of macrocyclic lactone (12, 14, 16 atom), the 12 membered ring macrolides are rarely used. The most prominent members of the 14 -atom rings are Erythromycin and Oleandomycin. Sixteen-membered macrolides are represented by Spiramycin. All macrocyclic lactone may contain ketone, hydroxyl, epoxide, olefin and glycosidically linked to one amino sugar and neutral sugar as shown in Erythromycin. the amino group provides a means to form the desirable water soluble salts, since the macrocyclic lactones are slightly soluble in water.

Macrolides bind to 50 S ribosomal subunit, inhibiting bacterial protein synthesis. The antibacterial activity of the macrolides mainly against gram-positive organisms and it is greater than that gram-negative organisms due to its superior penetration into gram-positive organism Macrolides used in the treatment of Legionella, Campylobacter, Chlamydia and Taxoplasma infections. Macrolides are mainly bacteriostatic, but has shown bactericidal activity in high concentration against very sensitive organisms

• Resistance to erythromycin is usually plasmid encoded. Three mechanisms have been identified: reduced permeability of the cell membrane or active efflux; production (by Enterobacteriaceae) of esterases that hydrolyze macrolides; and modification of the ribosomal binding site by chromosomal mutation or by a macrolide-inducible or a constitutive methylase. Efflux and methylase production account for the vast majority of cases of resistance in gram-positive organisms. Cross-resistance is complete between erythromycin and the other macrolides

Macrocyclic lactone Erythromycin under acidic condition is converted first to dihydrofuran then to spiroketal derivatives as presented below:

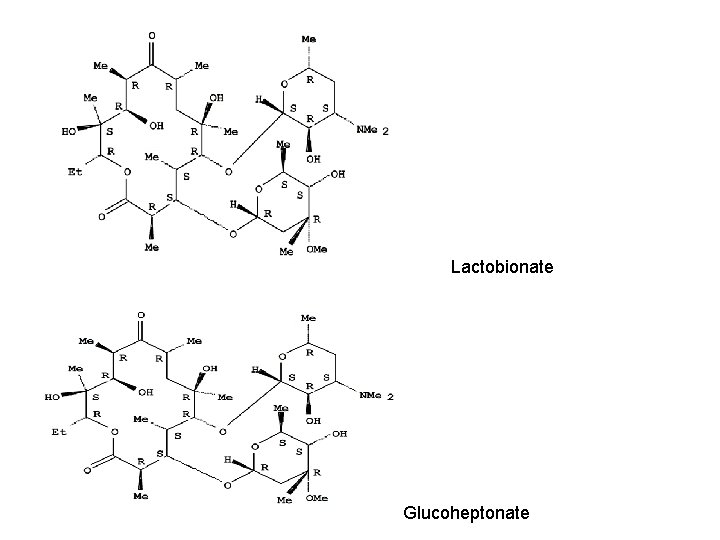
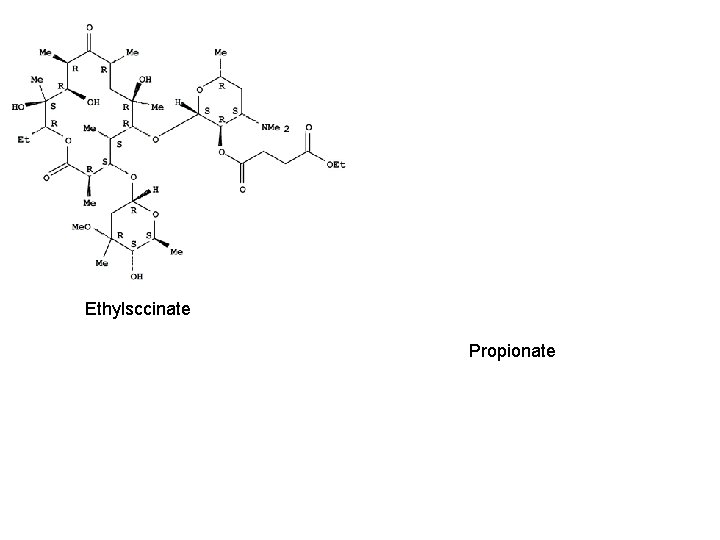
Erythromycin • • • Free base Stearate salt Glucoheptonate salt Lactobionate salt Ethylsuccinate ester Propionate ester

Lactobionate Glucoheptonate

Ethylsccinate Propionate

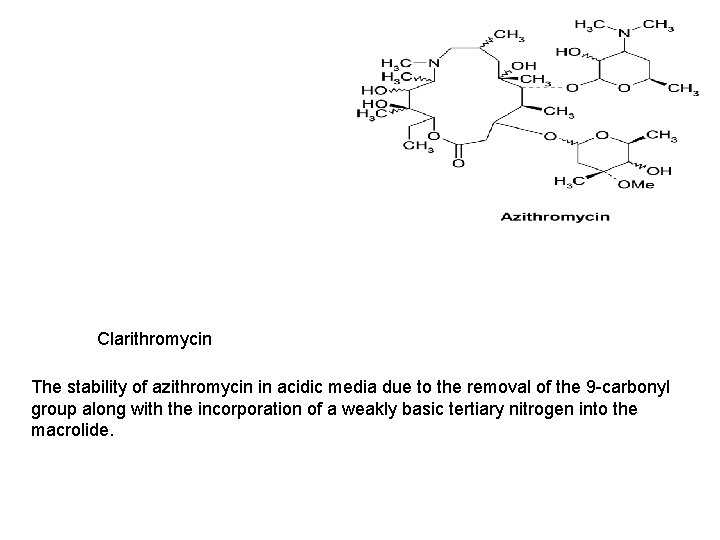
Clarithromycin The stability of azithromycin in acidic media due to the removal of the 9 -carbonyl group along with the incorporation of a weakly basic tertiary nitrogen into the macrolide.

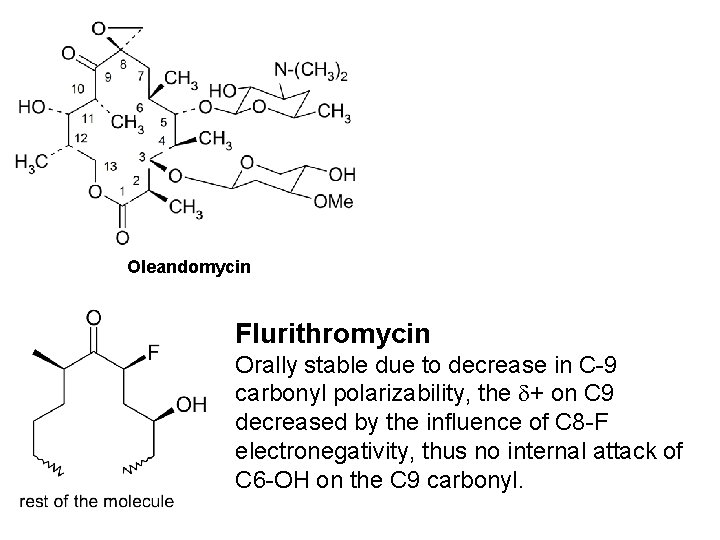
Oleandomycin Flurithromycin Orally stable due to decrease in C-9 carbonyl polarizability, the + on C 9 decreased by the influence of C 8 -F electronegativity, thus no internal attack of C 6 -OH on the C 9 carbonyl.

Telithromycin

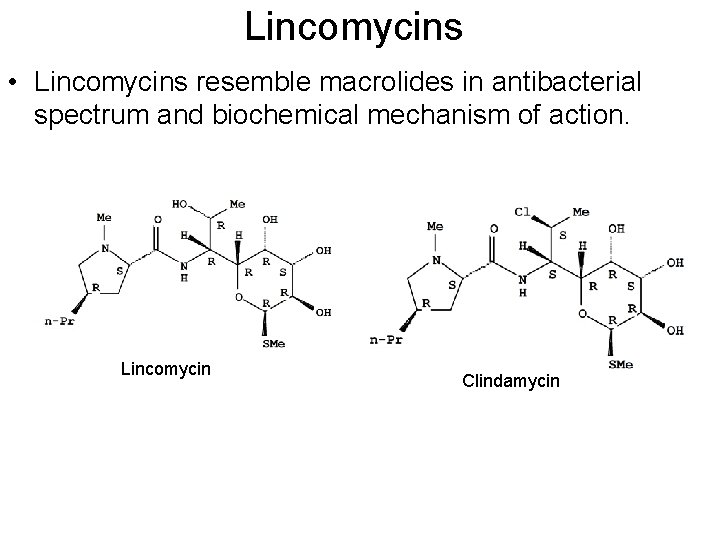
Lincomycins • Lincomycins resemble macrolides in antibacterial spectrum and biochemical mechanism of action. Lincomycin Clindamycin
 Lovastatin side effects
Lovastatin side effects Worksheet formulas consist of two components: operands and
Worksheet formulas consist of two components: operands and Servlet runs each request in a mcq
Servlet runs each request in a mcq What consists of the uk
What consists of the uk Consist of your most important targeted or segmented groups
Consist of your most important targeted or segmented groups Which are musical messages written around the brand
Which are musical messages written around the brand Diphthongs consist of
Diphthongs consist of A feeder consists of all circuit conductors located
A feeder consists of all circuit conductors located Scope of bureaucracy
Scope of bureaucracy Ipo chart java
Ipo chart java Direct view storage tube in computer graphics
Direct view storage tube in computer graphics Safety net drop tests must consist of a
Safety net drop tests must consist of a