Physics of Everyday Phenomena W Thomas Griffith Juliet




















- Slides: 20

Physics of Everyday Phenomena W. Thomas Griffith Juliet W. Brosing Chapter 10 Copyright © The Mc. Graw-Hill Companies, Inc. Permission required for reproduction or display.

Question 10. 1 At absolute zero A) all molecular motion is ceasing B) the pressure of an ideal gas is zero C) the volume of an ideal gas is zero D) the Celsius temperature is zero

Question 10. 2 The zeroth law of thermodynamics states that A) energy is conserved B) heat will flow from colder temperature to hotter temperature C) two objects in thermal equilibrium with each other have the same temperature D) the effect of 4. 19 Joule of work is the same as that of 1 calorie of heat in increasing the temperature of the system

Question 10. 3 When two objects at different temperatures are placed in thermal contact, heat flows A) only if both objects are at the same temperature B) from the higher temperature object to the lower temperature object C) from the lower temperature object to the higher temperature object

Question 10. 4 The amount of heat necessary to change the temperature of 1 kg of a substance by 1°C is called the substance's A) specific heat. B) latent heat. C) heat of combustion. D) mechanical equivalent of heat.

Question 10. 5 Latent heat is always A) part of the specific heat. B) related to the specific heat. C) the same as the mechanical equivalent of heat. D) involved in a phase change.

Question 10. 6 An amount of heat is added to ice, raising its temperature from -10°C to -5°C. A larger amount of heat is added to the same mass of liquid water, raising its temperature from 15°C to 20°C. From these results, we conclude that: A) overcoming the latent heat of fusion of ice requires an input of energy B) the latent heat of fusion of ice delivers some energy to the system C) the specific heat of ice is less than that of water D) the specific heat of ice is greater than that of water

Question 10. 7 Which is more likely to cause more damage to skin - a burn caused by water at 100 o. C or a burn caused by steam at 100 o. C? A) The burn caused by water. B) The burn caused by steam. C) There is no difference.

Question 10. 8 When water boils in Denver, Colorado (elevation 5, 200 ft) is its temperature greater than, less than, or equal to the temperature of boiling water in Dallas, Texas (elevation 450 ft). A) greater than B) less than C) equal to

Question 10. 9 The first law of thermodynamics states that the increase in internal energy of a system is equal to A) the amount of heat added to the system minus the amount of work done on the system B) the amount of heat added to the system plus the amount of work done on the system C) the amount of heat added to the system minus the amount of work done by the system D) the amount of heat added to the system minus the amount of work done on the system

Question 10. 10 When the temperature of a quantity of gas is increased A) the pressure must increase. B) the volume must increase. C) the pressure and/or the volume must increase. D) none of the above

Question 10. 11 The Kelvin temperature of an ideal gas is doubled and the volume is halved. How is the pressure affected? A) increases by a factor of 2 B) increases by a factor of 4 C) stays the same D) decreases by a factor of 2 E) decreases by a factor of 4

Question 10. 12 Why is the air at higher altitudes cooler than that at lower altitudes? A) Because of the relation expressed in Charles' Law, V/T = constant. B) Because of the relation expressed in Gay-Lussac's Law, P/T = constant. C) Because of the relation expressed in Boyle's Law, PV = constant.

Question 10. 13 Inside your apartment, you blow up a balloon as large as possible and then take it outside on a hot summer day. The balloon is most likely to then A) shrink. B) remain the same size. C) expand pop.

Question 10. 14 You fill your backpack with snacks for the long flight over to Europe on your summer vacation. When you take out your bag of chips mid-flight A) it looks just like it did when you packed it at home. B) it has shrunk and all of your chips are crushed. C) it has puffed up and looks like it could pop.

Question 10. 15 The heat transfer process in which heat flows directly through the material is A) conduction B) convection C) radiation

Question 10. 16 You are making macaroni and cheese for dinner, again. Which is the best choice for stirring the noodles in the pot of boiling water? A) a wooden spoon B) a metal spoon C) any kind of spoon D) your finger, but really fast

Question 10. 17 You are in the doctor's office and notice that the metal instrument tray feels much colder than the exam table you are sitting on. This is because A) the tray actually is colder than the table. B) the exam table has a cushion inside, which is a good insulator. C) the metal conducts heat away from your hand more quickly than does the table.

Question 18 Heating due to the greenhouse effect is caused by the fact that our atmosphere lets A) infrared wavelengths in but prevents heat loss at visible wavelengths B) visible light through but prevents heat loss at longer (infrared) wavelengths C) visible light through but prevents heat loss at shorter (ultraviolet) wavelengths

Answer Key to Chapter 10 1) 2) 3) 4) 5) 6) 7) 8) 9) A C B A D C B B C 10) 11) 12) 13) 14) 15) 16) 17) 18) C B B C C A A C B
 Griffith thomas
Griffith thomas Thomas mocker and thomas stewart
Thomas mocker and thomas stewart Moose monikko
Moose monikko Some natural phenomena class 8 ppt free download
Some natural phenomena class 8 ppt free download Physical and chemical phenomena
Physical and chemical phenomena Phenomena related to refraction
Phenomena related to refraction Observable phenomena
Observable phenomena Objective consideration of contemporary phenomena
Objective consideration of contemporary phenomena Natural language processing
Natural language processing Reference phenomenon in nlp
Reference phenomenon in nlp Colloids and interfaces
Colloids and interfaces Gravitation is a natural phenomenon where:
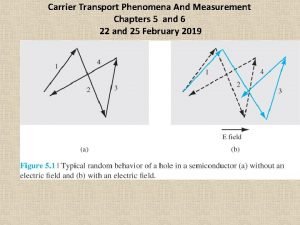
Gravitation is a natural phenomenon where: Carrier transport phenomena
Carrier transport phenomena Anchor phenomenon
Anchor phenomenon Observable phenomena
Observable phenomena Importance of interfacial phenomena in pharmacy
Importance of interfacial phenomena in pharmacy Reoulox phenomena
Reoulox phenomena Global climate phenomena
Global climate phenomena Random phenomena
Random phenomena Noumenon
Noumenon Sinusoidal functions in real life
Sinusoidal functions in real life