Phase Changes i e changes of state melting


- Slides: 11

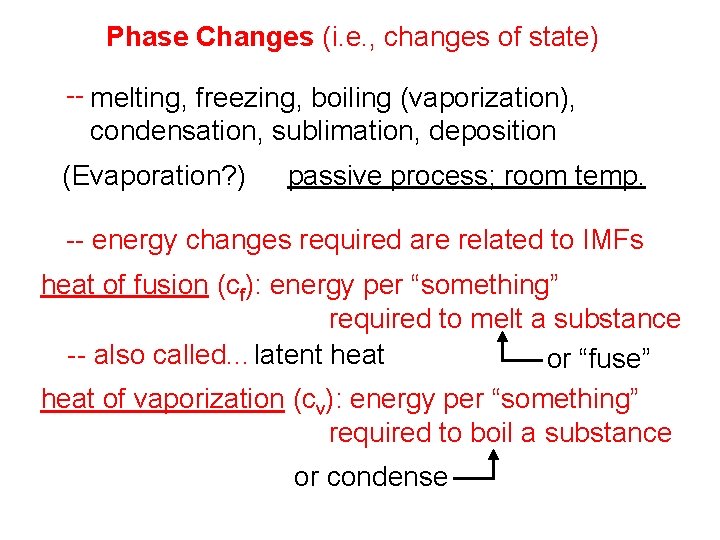
Phase Changes (i. e. , changes of state) -- melting, freezing, boiling (vaporization), condensation, sublimation, deposition (Evaporation? ) passive process; room temp. -- energy changes required are related to IMFs heat of fusion (cf): energy per “something” required to melt a substance -- also called. . . latent heat or “fuse” heat of vaporization (cv): energy per “something” required to boil a substance or condense

How do magnitudes of cv and > cf compare? KE must be increased IMFs must be enough to allow completely overcome particles to slide, relative (i. e. , “broken”) to each other (IMFs still in effect)

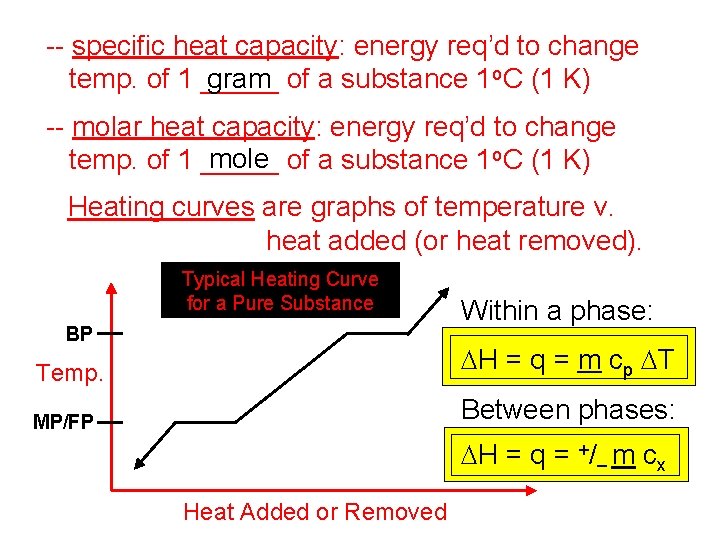
-- specific heat capacity: energy req’d to change temp. of 1 _____ gram of a substance 1 o. C (1 K) -- molar heat capacity: energy req’d to change mole of a substance 1 o. C (1 K) temp. of 1 _____ Heating curves are graphs of temperature v. heat added (or heat removed). Typical Heating Curve for a Pure Substance BP Within a phase: Temp. DH = q = m cp DT MP/FP Between phases: DH = q = +/– m cx Heat Added or Removed

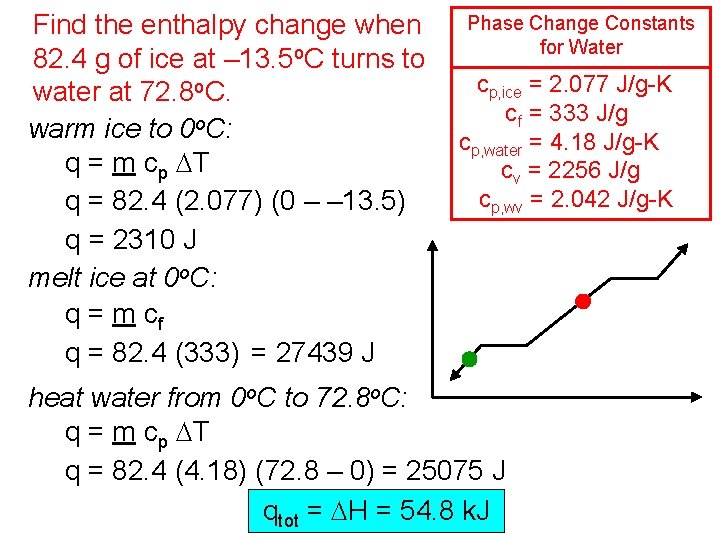
Find the enthalpy change when 82. 4 g of ice at – 13. 5 o. C turns to water at 72. 8 o. C. warm ice to 0 o. C: q = m cp DT q = 82. 4 (2. 077) (0 – – 13. 5) q = 2310 J melt ice at 0 o. C: q = m cf q = 82. 4 (333) = 27439 J Phase Change Constants for Water cp, ice = 2. 077 J/g-K cf = 333 J/g cp, water = 4. 18 J/g-K cv = 2256 J/g cp, wv = 2. 042 J/g-K heat water from 0 o. C to 72. 8 o. C: q = m cp DT q = 82. 4 (4. 18) (72. 8 – 0) = 25075 J qtot = DH = 54. 8 k. J

supercooling: temporarily cooling a liquid below its freezing point without it forming a solid -- heat is removed so quickly that particles have no time to assume an ordered structure critical temperature: the highest temperature at which a substance can be a liquid -- as IMFs increase, crit. temp… increases critical pressure: the pressure required to bring about liquefaction at the critical temp. The intersection of the critical temperature and the critical pressure is called the critical point.

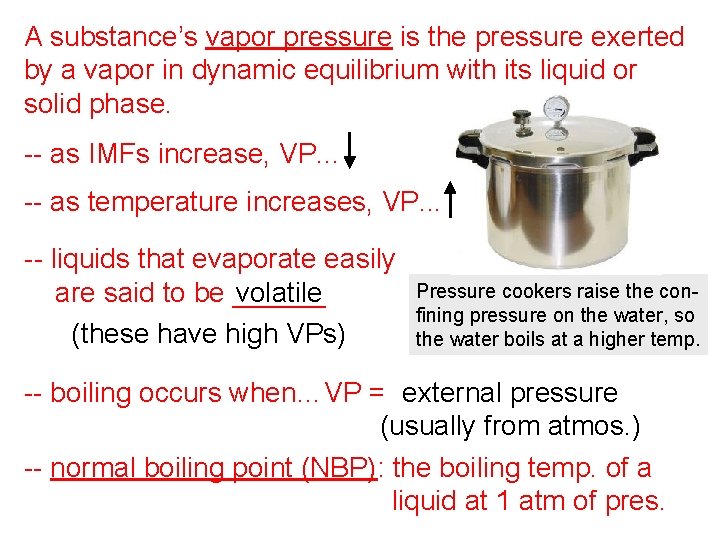
A substance’s vapor pressure is the pressure exerted by a vapor in dynamic equilibrium with its liquid or solid phase. -- as IMFs increase, VP. . . -- as temperature increases, VP. . . -- liquids that evaporate easily are said to be ______ volatile (these have high VPs) Pressure cookers raise the confining pressure on the water, so the water boils at a higher temp. -- boiling occurs when…VP = external pressure (usually from atmos. ) -- normal boiling point (NBP): the boiling temp. of a liquid at 1 atm of pres.

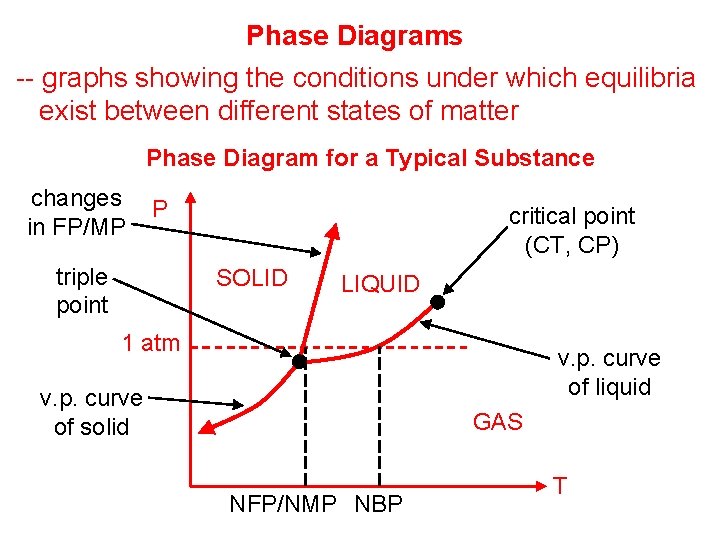
Phase Diagrams -- graphs showing the conditions under which equilibria exist between different states of matter Phase Diagram for a Typical Substance changes in FP/MP P triple point critical point (CT, CP) SOLID LIQUID 1 atm v. p. curve of liquid v. p. curve of solid GAS NFP/NMP NBP T

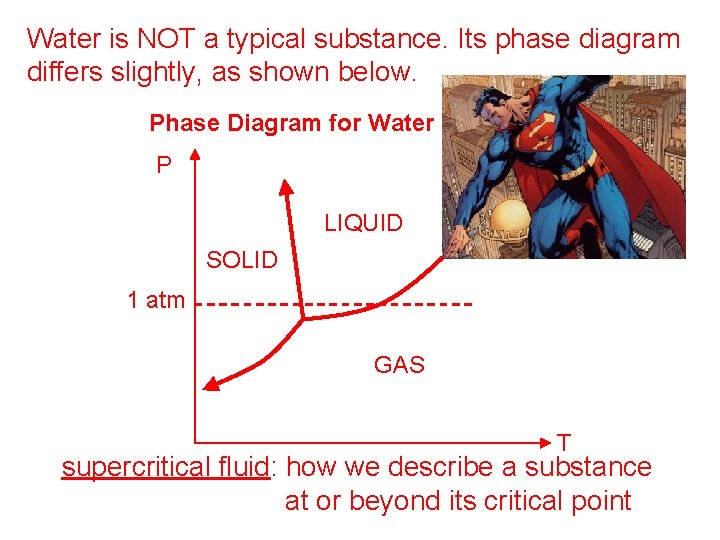
Water is NOT a typical substance. Its phase diagram differs slightly, as shown below. Phase Diagram for Water P LIQUID s. c. f. SOLID 1 atm GAS T supercritical fluid: how we describe a substance at or beyond its critical point

Structures of Solids amorphous solid: the particles have no orderly structure -- e. g. , rubber, glass -- IMFs are highly variable, so these solids have no specific… MP

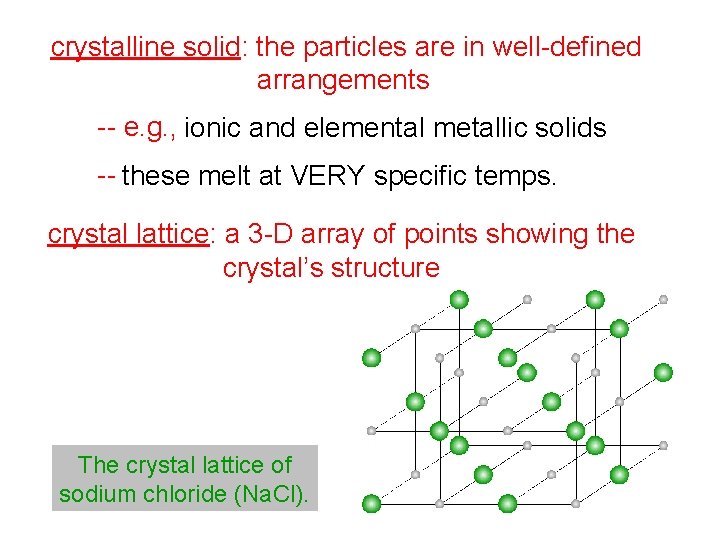
crystalline solid: the particles are in well-defined arrangements -- e. g. , ionic and elemental metallic solids -- these melt at VERY specific temps. crystal lattice: a 3 -D array of points showing the crystal’s structure The crystal lattice of sodium chloride (Na. Cl).

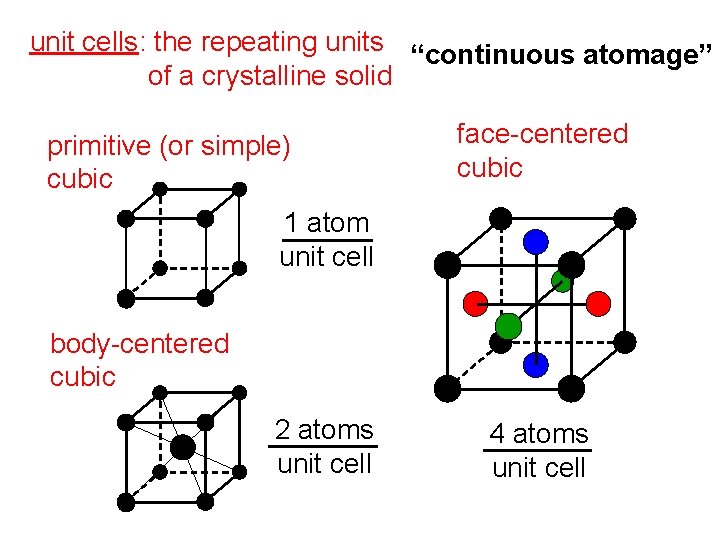
unit cells: the repeating units “continuous atomage” of a crystalline solid primitive (or simple) cubic face-centered cubic 1 atom unit cell body-centered cubic 2 atoms unit cell 4 atoms unit cell