Changes of State Phase Changes Phase Change A









- Slides: 17


Changes of State (Phase Changes)

Phase Change • A phase change is going from one state of matter to another (Physical change) – Gas to liquid – Liquid to solid • Changes of state either absorb or release (evolve/liberate) energy

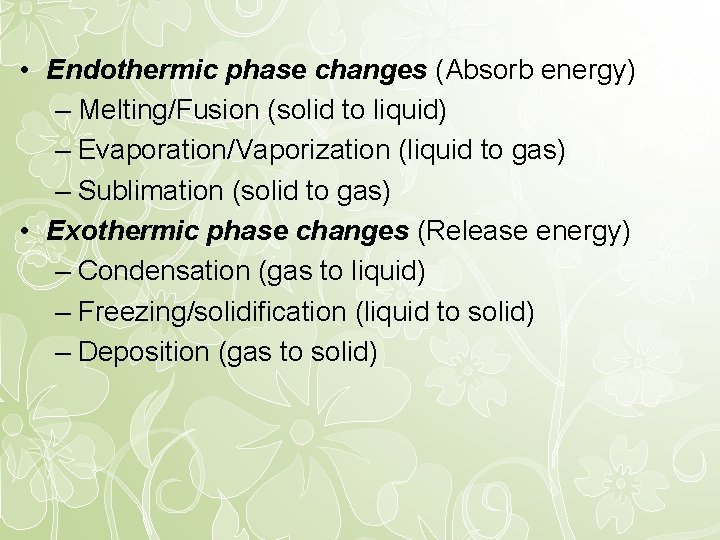
• Endothermic phase changes (Absorb energy) – Melting/Fusion (solid to liquid) – Evaporation/Vaporization (liquid to gas) – Sublimation (solid to gas) • Exothermic phase changes (Release energy) – Condensation (gas to liquid) – Freezing/solidification (liquid to solid) – Deposition (gas to solid)


Temperature and Phase Change • During a phase change, as energy is added or removed, there is no temperature change.

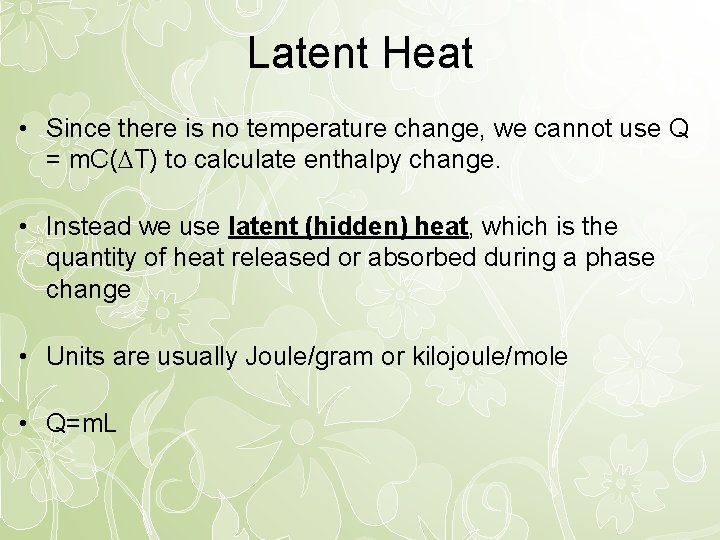
Latent Heat • Since there is no temperature change, we cannot use Q = m. C( T) to calculate enthalpy change. • Instead we use latent (hidden) heat, which is the quantity of heat released or absorbed during a phase change • Units are usually Joule/gram or kilojoule/mole • Q=m. L

Changes of State • Latent Heat of Vaporization is the heat required to vaporize one mole of a liquid – Going from liquid to gas – For water, Hvap = 40. 7 k. J/mol • Latent Heat of Condensation is the heat required to condense one mole of a gas – Going from gas to liquid – For water, Hcond = -40. 7 k. J/mol

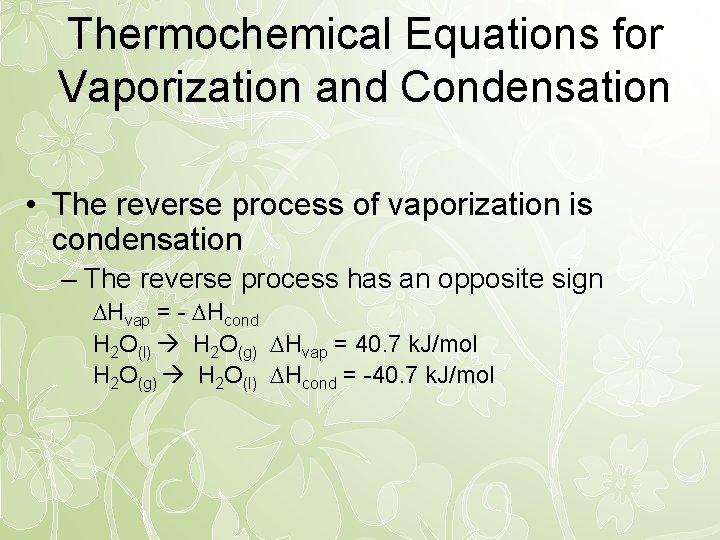
Thermochemical Equations for Vaporization and Condensation • The reverse process of vaporization is condensation – The reverse process has an opposite sign Hvap = - Hcond H 2 O(l) H 2 O(g) Hvap = 40. 7 k. J/mol H 2 O(g) H 2 O(l) Hcond = -40. 7 k. J/mol

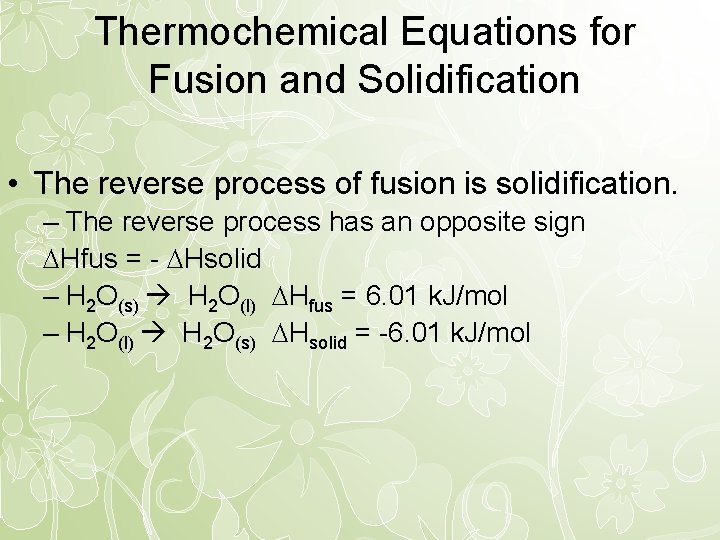
Thermochemical Equations for Fusion and Solidification • The reverse process of fusion is solidification. – The reverse process has an opposite sign Hfus = - Hsolid – H 2 O(s) H 2 O(l) Hfus = 6. 01 k. J/mol – H 2 O(l) H 2 O(s) Hsolid = -6. 01 k. J/mol

Changes of State • Latent Heat of fusion is the heat required to melt one mole of a solid – Going from solid to liquid – For water, Hfus = 6. 1 k. J/mol • Latent Heat of solidification is the heat required to solidify one mole of a liquid – Going from liquid to solid – For water, Hsolid = -6. 1 k. J/mol

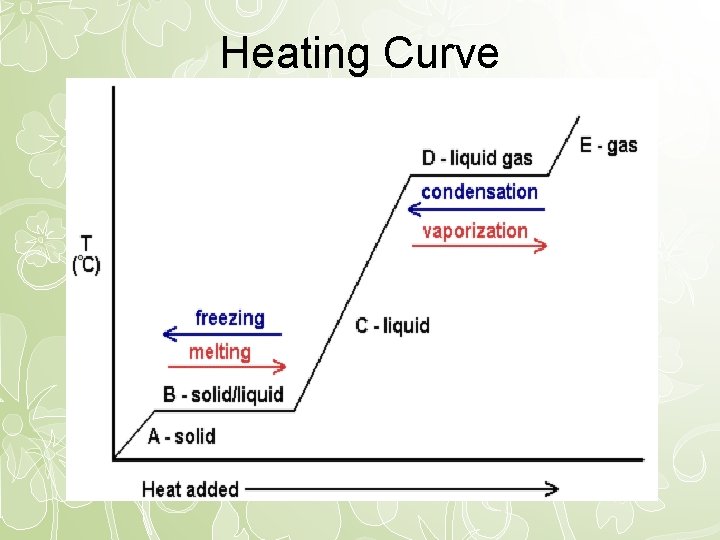
Heating Curve

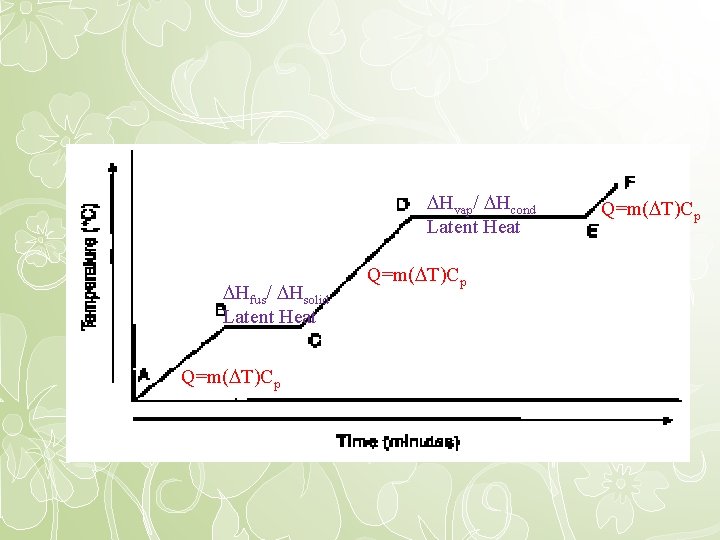
Explanation of Heating Curve • When there is a temperature change use Q=m. C( T) to calculate the amount of energy used. • When the heating curve is flat (no temperature change), there is a phase change. Use the latent heat to calculate the amount of energy used. Q=m. L

Hvap/ Hcond Latent Heat Hfus/ Hsolid Latent Heat Q=m( T)Cp

Example 1 Calculate the heat required to melt 45. 6 g of water at its melting point. Latent heat of fusion (∆Hfus)=6. 01 k. J/mol

Example 2 Calculate the heat evolved to condense 75. 4 g of water at its boiling point. Latent heat of vaporization (∆Hvap)= 40. 7 k. J/mol

Example 3 What mass of ammonia (NH 3) will vaporize when 345 k. J of heat is absorbed? Latent heat of vaporization (∆Hvap)=23. 3 k. J/mol