Patient Selection and TAVR VS SAVR Are we




- Slides: 28

Patient Selection and TAVR VS. SAVR Are we Ready for Low-Risk Patients? Ron Waksman, MD, FACC, FSCAI Professor of Medicine, (Cardiology) Georgetown University Director, Cardiovascular Research Advanced Education, Med. Star Heart & Vascular Institute, Washington DC

Disclosure Statement of Financial Interest Within the past 12 months, I or my spouse/partner have had a financial interest/arrangement or affiliation with the organization(s) listed below. Affiliation/Financial Relationship • Grant/Research Support • Consulting Fees/Honoraria Company • Boston Scientific • Biotronik • Biosensors • Astra Zeneca • Medtronic Vascular • Abbott Vascular • Symetis • Med Alliance • Life. Tech • Amgen

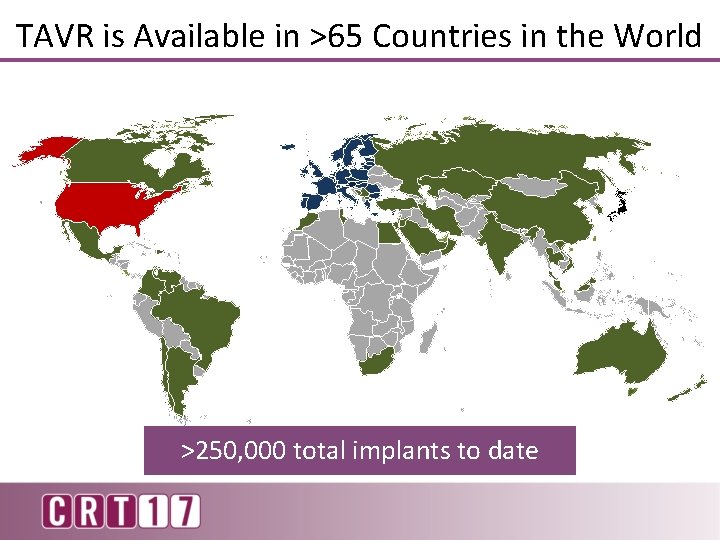
TAVR is Available in >65 Countries in the World >250, 000 total implants to date

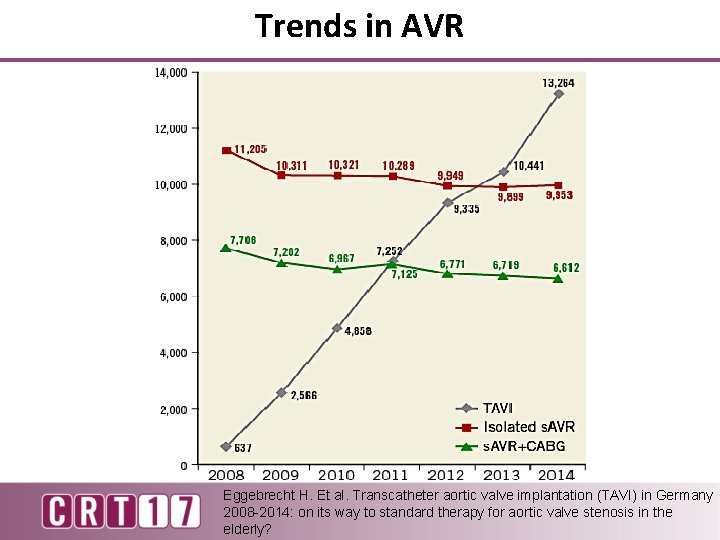
Trends in AVR Eggebrecht H. Et al. Transcatheter aortic valve implantation (TAVI) in Germany 2008 -2014: on its way to standard therapy for aortic valve stenosis in the elderly?

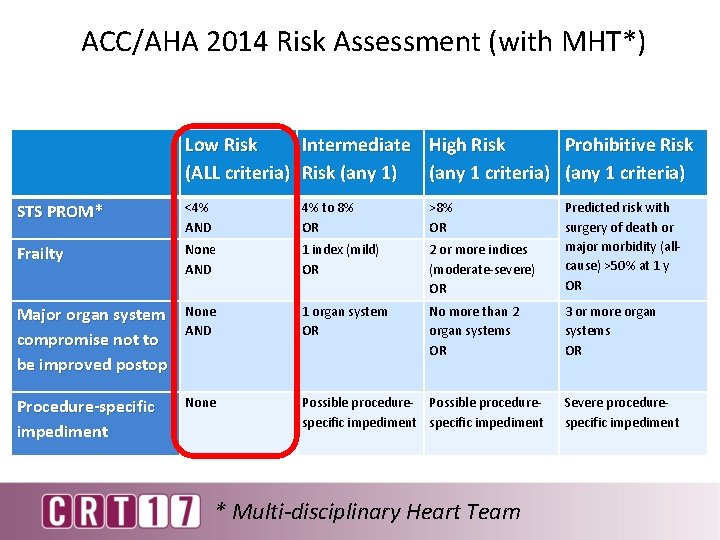
ACC/AHA 2014 Risk Assessment (with MHT*) Low Risk Intermediate High Risk Prohibitive Risk (ALL criteria) Risk (any 1) (any 1 criteria) STS PROM* <4% AND 4% to 8% OR >8% OR Frailty None AND 1 index (mild) OR 2 or more indices (moderate-severe) OR Predicted risk with surgery of death or major morbidity (allcause) >50% at 1 y OR Major organ system compromise not to be improved postop None AND 1 organ system OR No more than 2 organ systems OR 3 or more organ systems OR Procedure-specific impediment None Possible procedure- Possible procedurespecific impediment * Multi-disciplinary Heart Team Severe procedurespecific impediment

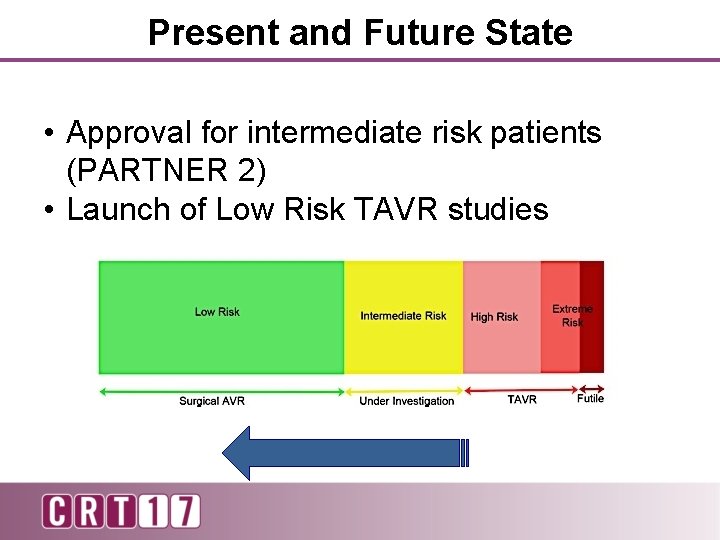
Present and Future State • Approval for intermediate risk patients (PARTNER 2) • Launch of Low Risk TAVR studies

TAVR in Low-Risk Patients State of the Field • TAVR is now the preferred therapy in AS patients in… • Inoperable • High-risk • intermediate-risk • Secondary benefits associated with TAVR over SAVR • Reduced ICU and hospital LOS • More rapid Qo. L recovery • Lower frequency of AKI, bleeding, and post-operative AF • Improved valve hemodynamics • TAVR is now being studied in low-risk AS patients in randomized and propensity-matched clinical trials!

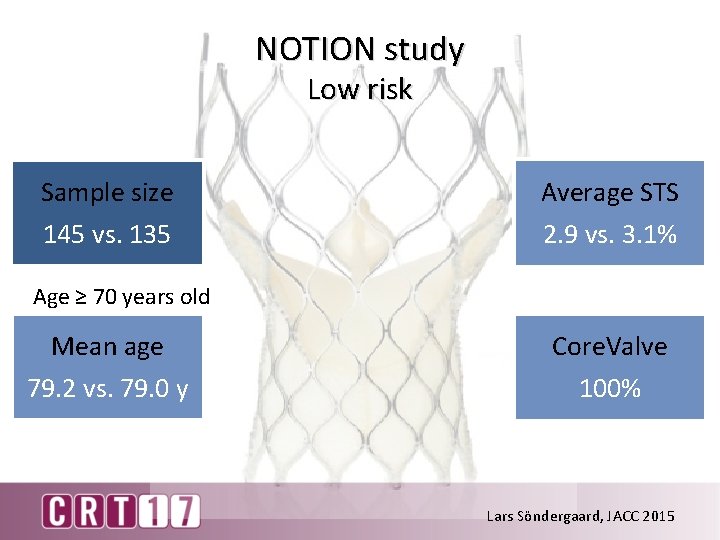
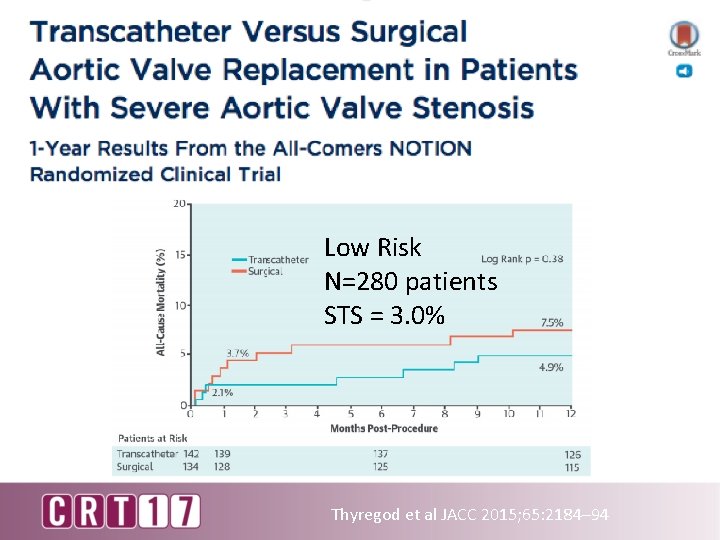
NOTION study Low risk Sample size 145 vs. 135 Average STS 2. 9 vs. 3. 1% Age ≥ 70 years old Mean age 79. 2 vs. 79. 0 y Core. Valve 100% Lars Söndergaard, JACC 2015

Low Risk N=280 patients STS = 3. 0% Thyregod et al JACC 2015; 65: 2184– 94

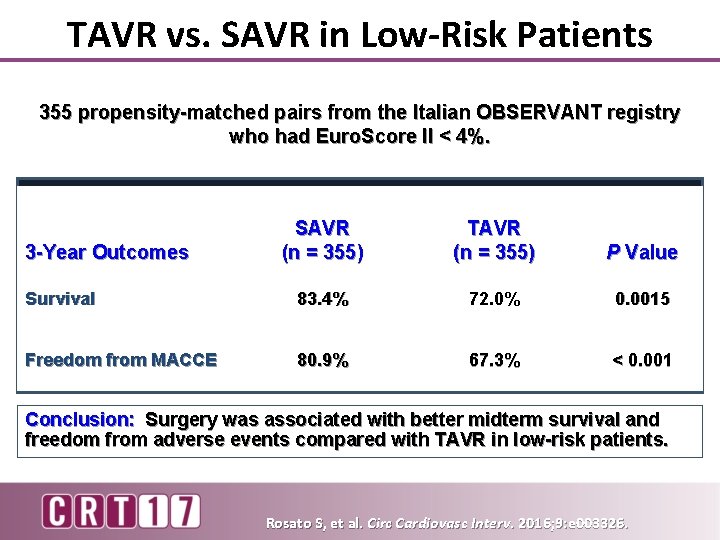
TAVR vs. SAVR in Low-Risk Patients 355 propensity-matched pairs from the Italian OBSERVANT registry who had Euro. Score II < 4%. SAVR (n = 355) TAVR (n = 355) P Value Survival 83. 4% 72. 0% 0. 0015 Freedom from MACCE 80. 9% 67. 3% < 0. 001 3 -Year Outcomes Conclusion: Surgery was associated with better midterm survival and freedom from adverse events compared with TAVR in low-risk patients. Rosato S, et al. Circ Cardiovasc Interv. 2016; 9: e 003326.

Sponsor: Med. Star Health Investigator Sponsor Study National Co-Principal Investigators: Ron Waksman, MD Paul Corso, MD First Low Risk TAVR study in the US!

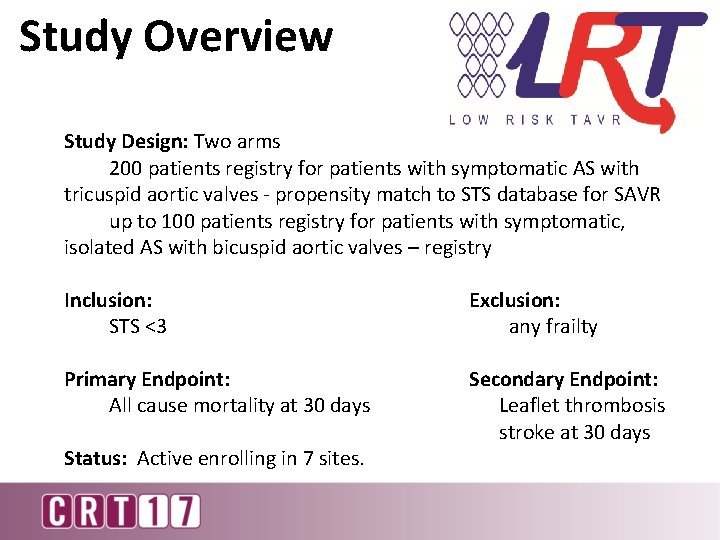
Study Overview Study Design: Two arms 200 patients registry for patients with symptomatic AS with tricuspid aortic valves - propensity match to STS database for SAVR up to 100 patients registry for patients with symptomatic, isolated AS with bicuspid aortic valves – registry Inclusion: STS <3 Exclusion: any frailty Primary Endpoint: All cause mortality at 30 days Secondary Endpoint: Leaflet thrombosis stroke at 30 days Status: Active enrolling in 7 sites.

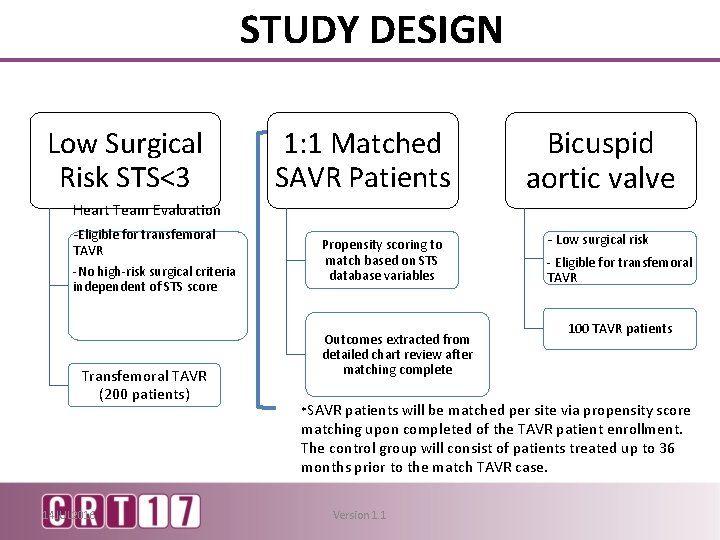
STUDY DESIGN Low Surgical Risk STS<3 Heart Team Evaluation -Eligible for transfemoral TAVR -No high-risk surgical criteria independent of STS score Transfemoral TAVR (200 patients) 14 JUL 2016 1: 1 Matched SAVR Patients Propensity scoring to match based on STS database variables Outcomes extracted from detailed chart review after matching complete *SAVR Bicuspid aortic valve - Low surgical risk - Eligible for transfemoral TAVR 100 TAVR patients will be matched per site via propensity score matching upon completed of the TAVR patient enrollment. The control group will consist of patients treated up to 36 months prior to the match TAVR case. Version 1. 1

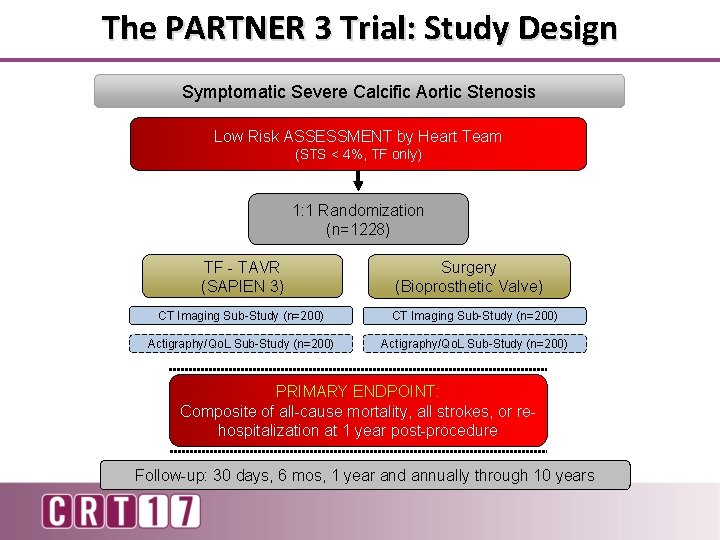
The PARTNER 3 Trial: Study Design Symptomatic Severe Calcific Aortic Stenosis Low Risk ASSESSMENT by Heart Team (STS < 4%, TF only) 1: 1 Randomization (n=1228) TF - TAVR (SAPIEN 3) Surgery (Bioprosthetic Valve) CT Imaging Sub-Study (n=200) Actigraphy/Qo. L Sub-Study (n=200) PRIMARY ENDPOINT: Composite of all-cause mortality, all strokes, or rehospitalization at 1 year post-procedure Follow-up: 30 days, 6 mos, 1 year and annually through 10 years

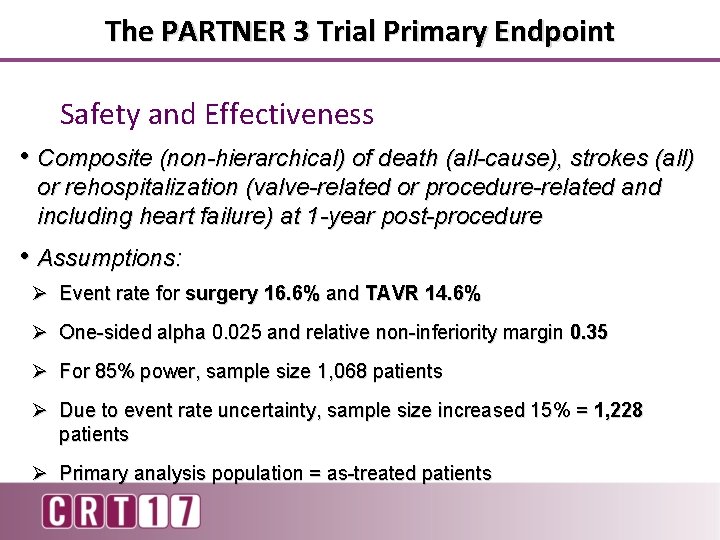
The PARTNER 3 Trial Primary Endpoint Safety and Effectiveness • Composite (non-hierarchical) of death (all-cause), strokes (all) or rehospitalization (valve-related or procedure-related and including heart failure) at 1 -year post-procedure • Assumptions: Ø Event rate for surgery 16. 6% and TAVR 14. 6% Ø One-sided alpha 0. 025 and relative non-inferiority margin 0. 35 Ø For 85% power, sample size 1, 068 patients Ø Due to event rate uncertainty, sample size increased 15% = 1, 228 patients Ø Primary analysis population = as-treated patients

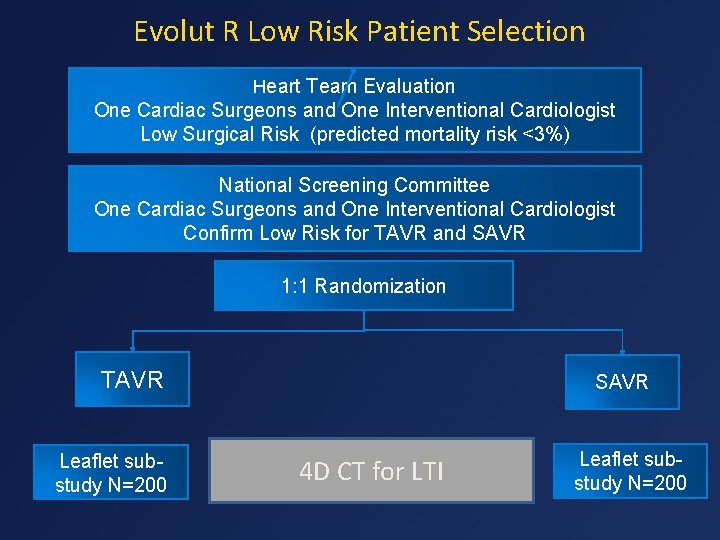
Evolut R Low Risk Patient Selection Heart Team Evaluation One Cardiac Surgeons and One Interventional Cardiologist Low Surgical Risk (predicted mortality risk <3%) National Screening Committee One Cardiac Surgeons and One Interventional Cardiologist Confirm Low Risk for TAVR and SAVR 1: 1 Randomization TAVR Leaflet substudy N=200 SAVR 4 D CT for LTI Leaflet substudy N=200

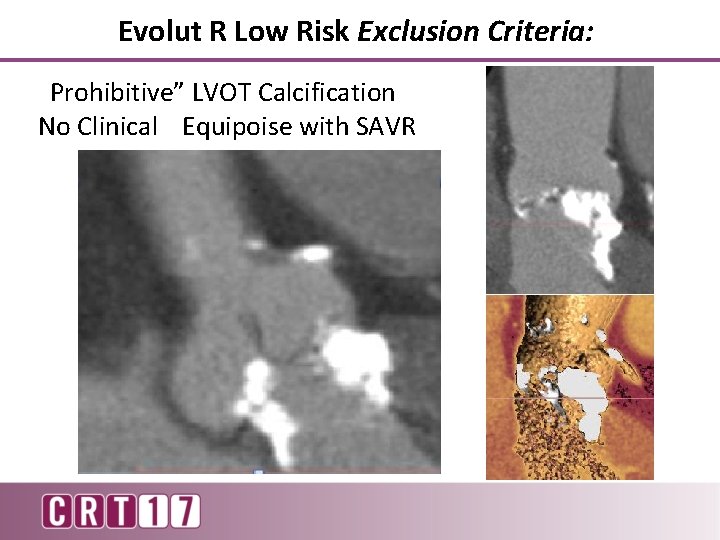
Evolut R Low Risk Exclusion Criteria: “Prohibitive” LVOT Calcification No Clinical Equipoise with SAVR

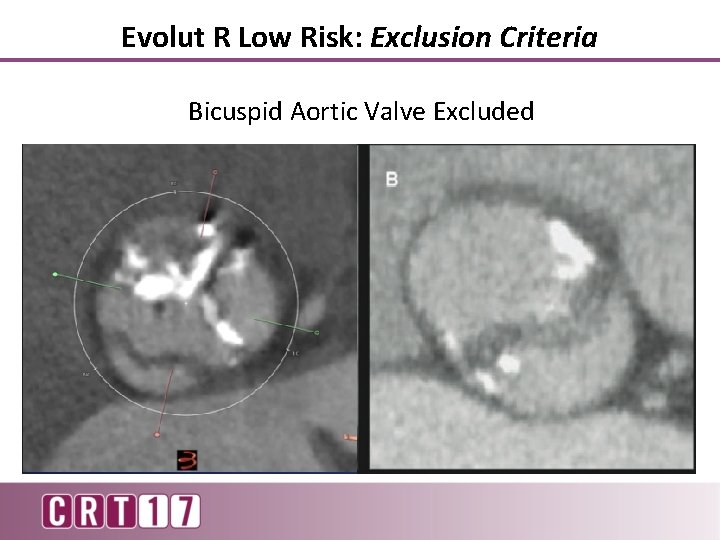
Evolut R Low Risk: Exclusion Criteria Bicuspid Aortic Valve Excluded

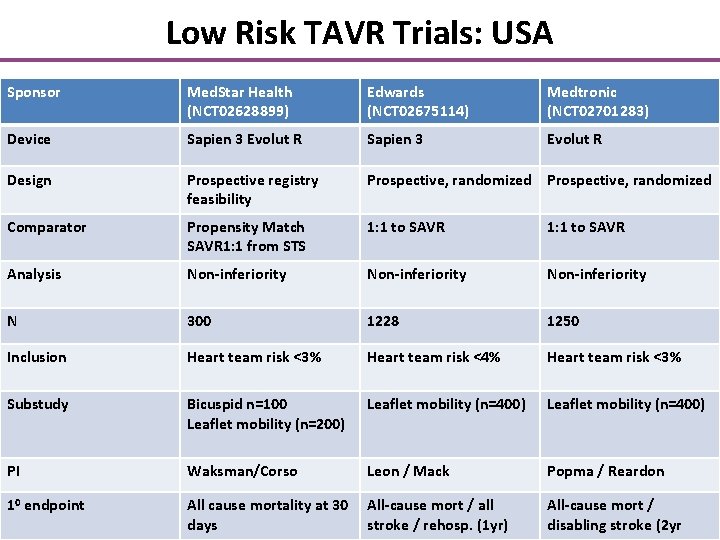
Low Risk TAVR Trials: USA Sponsor Med. Star Health (NCT 02628899) Edwards (NCT 02675114) Medtronic (NCT 02701283) Device Sapien 3 Evolut R Design Prospective registry feasibility Prospective, randomized Comparator Propensity Match SAVR 1: 1 from STS 1: 1 to SAVR Analysis Non-inferiority N 300 1228 1250 Inclusion Heart team risk <3% Heart team risk <4% Heart team risk <3% Substudy Bicuspid n=100 Leaflet mobility (n=200) Leaflet mobility (n=400) PI Waksman/Corso Leon / Mack Popma / Reardon 10 endpoint All cause mortality at 30 All-cause mort / all days stroke / rehosp. (1 yr) All-cause mort / disabling stroke (2 yr

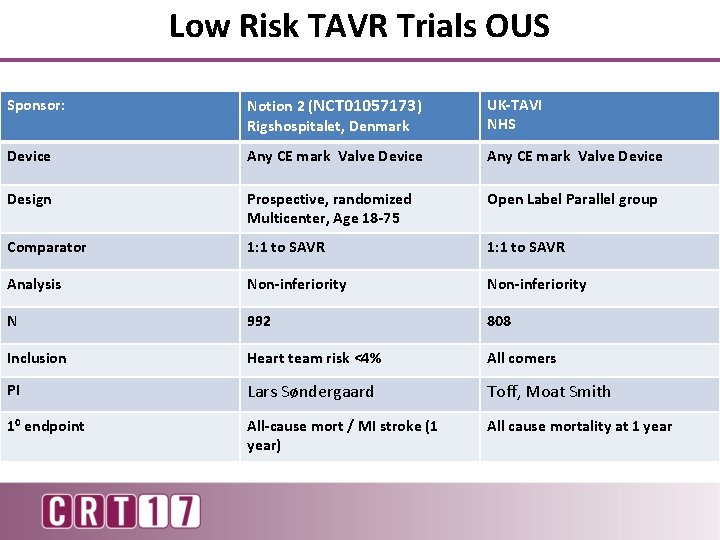
Low Risk TAVR Trials OUS Sponsor: Notion 2 (NCT 01057173) Rigshospitalet, Denmark UK-TAVI NHS Device Any CE mark Valve Device Design Prospective, randomized Multicenter, Age 18 -75 Open Label Parallel group Comparator 1: 1 to SAVR Analysis Non-inferiority N 992 808 Inclusion Heart team risk <4% All comers PI Lars Søndergaard Toff, Moat Smith 10 endpoint All-cause mort / MI stroke (1 year) All cause mortality at 1 year

Enrollment Challenges: Low Risk Studies • Patients do not want to be randomized, results in shifting from low risk to intermediate risk • Contemporary clinical practice has already evolved in the EU and in the U. S. – risk strata have already “downshifted” to include Low risk patients even before clinical trial data available • Surgeons reluctant to randomized their patients • Industry does not allow competing studies per center

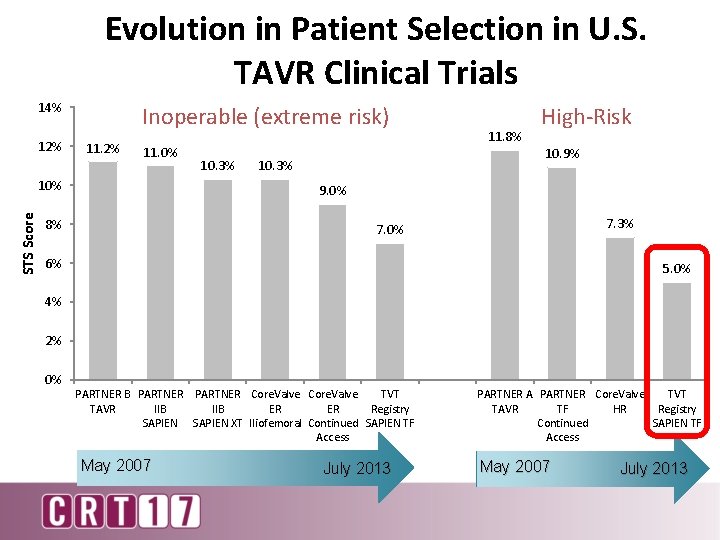
Evolution in Patient Selection in U. S. TAVR Clinical Trials 14% 12% Inoperable (extreme risk) 11. 2% 11. 0% STS Score 10% 10. 3% 11. 8% High-Risk 10. 9% 10. 3% 9. 0% 8% 7. 3% 7. 0% 6% 5. 0% 4% 2% 0% PARTNER B PARTNER Core. Valve TVT TAVR IIB ER ER Registry SAPIEN XT Iliofemoral Continued SAPIEN TF Access May 2007 July 2013 PARTNER A PARTNER Core. Valve TVT TAVR TF HR Registry Continued SAPIEN TF Access May 2007 July 2013

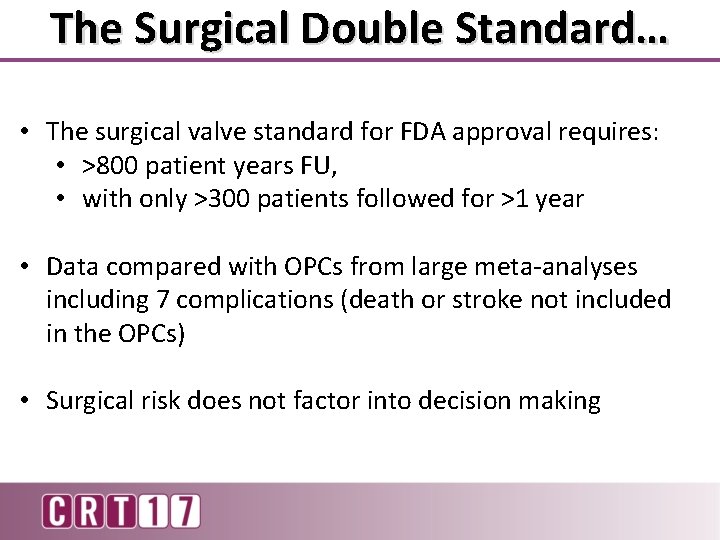
The Surgical Double Standard… • The surgical valve standard for FDA approval requires: • >800 patient years FU, • with only >300 patients followed for >1 year • Data compared with OPCs from large meta-analyses including 7 complications (death or stroke not included in the OPCs) • Surgical risk does not factor into decision making

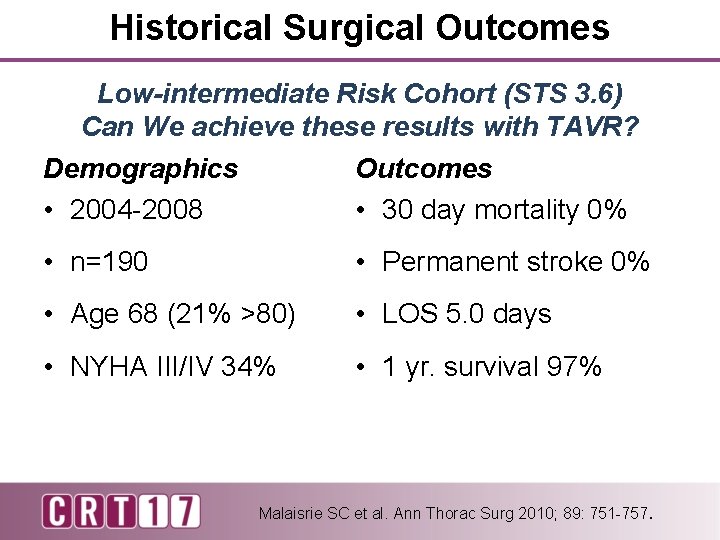
Historical Surgical Outcomes Low-intermediate Risk Cohort (STS 3. 6) Can We achieve these results with TAVR? Demographics • 2004 -2008 Outcomes • 30 day mortality 0% • n=190 • Permanent stroke 0% • Age 68 (21% >80) • LOS 5. 0 days • NYHA III/IV 34% • 1 yr. survival 97% Malaisrie SC et al. Ann Thorac Surg 2010; 89: 751 -757.

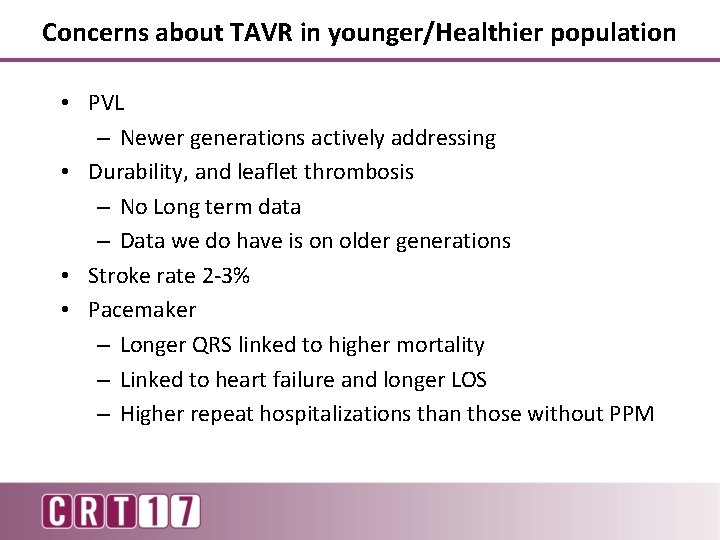
Concerns about TAVR in younger/Healthier population • PVL – Newer generations actively addressing • Durability, and leaflet thrombosis – No Long term data – Data we do have is on older generations • Stroke rate 2 -3% • Pacemaker – Longer QRS linked to higher mortality – Linked to heart failure and longer LOS – Higher repeat hospitalizations than those without PPM

What does TAVR have to demonstrate to treat low risk patients? ØExcellent procedural outcomes ØGood hemodynamic results ØLong-term durability ØTo be cost neutral to SAVR

Summary & Conclusions As of today in 2017… • 3 RCTs and one registry of low risk TAVR • N ~ 3400 patients • Similar but not identical protocols, endpoints • Recruitment challenges; equipoise • Short- vs. long-term follow up • The bar is high; the trials are well designed

Thank You for Your Attention
 Mikael ferm
Mikael ferm Annular rupture tavr
Annular rupture tavr Tavr antiplatelet guidelines
Tavr antiplatelet guidelines Calgene flavr savr tomato
Calgene flavr savr tomato Flavr savr tomato
Flavr savr tomato Flavr savr pronunciation
Flavr savr pronunciation Patient 2 patient
Patient 2 patient Two way selection and multiway selection
Two way selection and multiway selection Multiway selection in c
Multiway selection in c Procedure of pure line selection
Procedure of pure line selection Appropriate patient selection
Appropriate patient selection Balancing selection vs stabilizing selection
Balancing selection vs stabilizing selection Similarities
Similarities K selection r selection
K selection r selection Natural selection vs artificial selection
Natural selection vs artificial selection Difference between continuous and discontinuous variation
Difference between continuous and discontinuous variation What is naturual selection
What is naturual selection What is exponential growth in ecology
What is exponential growth in ecology Natural selection vs artificial selection
Natural selection vs artificial selection Recruitment selection and induction policies and procedures
Recruitment selection and induction policies and procedures Maintenance of records and reports in obg
Maintenance of records and reports in obg Pico question for falls and hourly rounding
Pico question for falls and hourly rounding Primary nursing care
Primary nursing care Geisingerconnect
Geisingerconnect Bedside shift report and patient satisfaction
Bedside shift report and patient satisfaction Chapter 8 body mechanics and patient mobility
Chapter 8 body mechanics and patient mobility A nurse floats to a busy surgical unit
A nurse floats to a busy surgical unit Chapter 20 patient environment and safety
Chapter 20 patient environment and safety Chapter 20 patient collections and financial management
Chapter 20 patient collections and financial management