Suggested guidelines for appropriate patient selection for patients











- Slides: 15

Suggested guidelines for appropriate patient selection for patients undergoing Accelerated Partial Breast Irradiation at DMC. Tonya Echols Cole, MD

Patient Evaluation • Multidisciplinary; before surgery – Breast Surgeon – Radiation Oncologist – Medical Oncologists

Types of APBI • Interstitial • Intraoperative – Electron – 50 Kv • Balloon cavitary – Mammosite – Contura – Savi • External Beam – 3 D conformal – IMRT

Current Guidelines- Old • • • ASBS - 2005 ABS - 2007 European Collaborative Group - 2009 ACRO - 2008 ASTRO- 2009

Additional Studies • At least 5 randomized trials – Short follow-up – Outdated or nonstandard technique – Lack of power • At least 41 non randomized trials – Many with at least 10 year follow-up

Clinical Trial Results • ASBS Mammosite Registry Trial – 44 month follow-up on first 400 cases. – Cancer specific survival of 100% – Local recurrence rate between 0 -2. 65% • DCIS Phase II Clinical Study – 15 month follow-up on 100 patients – 3% local recurrence rate

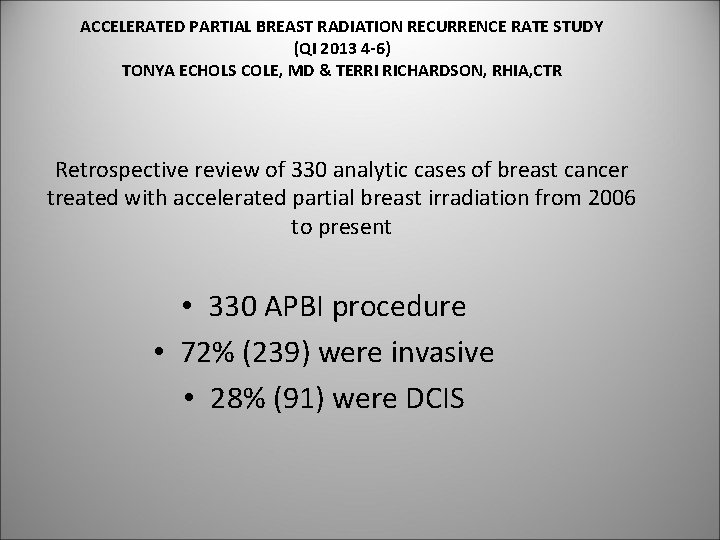
ACCELERATED PARTIAL BREAST RADIATION RECURRENCE RATE STUDY (QI 2013 4 -6) TONYA ECHOLS COLE, MD & TERRI RICHARDSON, RHIA, CTR Retrospective review of 330 analytic cases of breast cancer treated with accelerated partial breast irradiation from 2006 to present • 330 APBI procedure • 72% (239) were invasive • 28% (91) were DCIS

Recurrences • • • 14 total recurrences ( 4. 2%) 7 Local (2. 1%) 3 Regional (0. 9%) 4 Distant (1. 2%) Median time to recurrence was 53 months

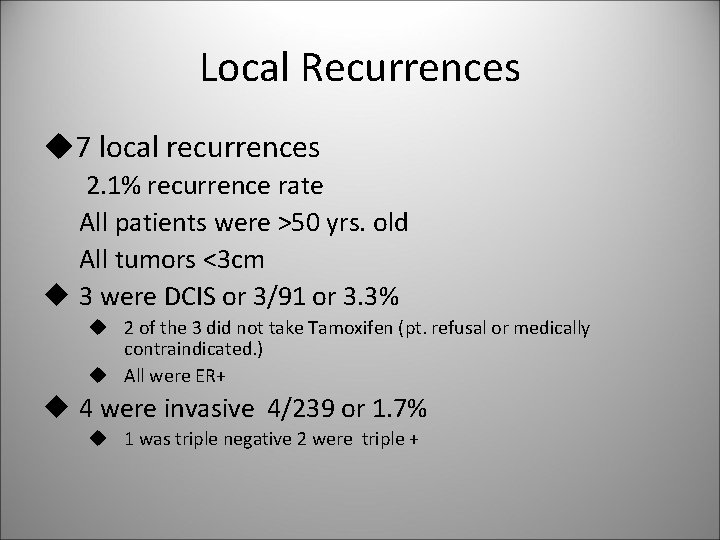
Local Recurrences u 7 local recurrences 2. 1% recurrence rate All patients were >50 yrs. old All tumors <3 cm u 3 were DCIS or 3/91 or 3. 3% u 2 of the 3 did not take Tamoxifen (pt. refusal or medically contraindicated. ) u All were ER+ u 4 were invasive 4/239 or 1. 7% u 1 was triple negative 2 were triple +

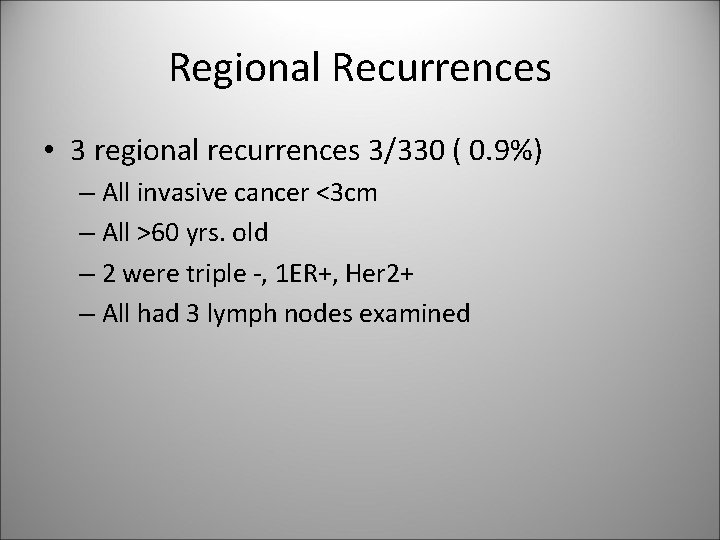
Regional Recurrences • 3 regional recurrences 3/330 ( 0. 9%) – All invasive cancer <3 cm – All >60 yrs. old – 2 were triple -, 1 ER+, Her 2+ – All had 3 lymph nodes examined

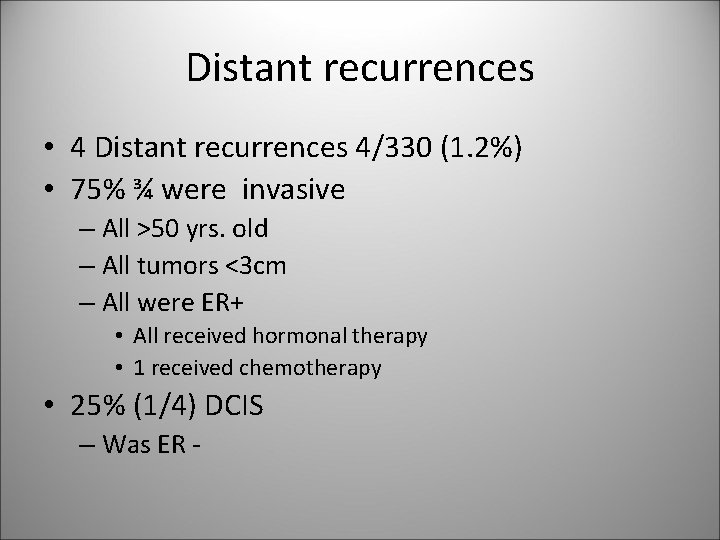
Distant recurrences • 4 Distant recurrences 4/330 (1. 2%) • 75% ¾ were invasive – All >50 yrs. old – All tumors <3 cm – All were ER+ • All received hormonal therapy • 1 received chemotherapy • 25% (1/4) DCIS – Was ER -

Conclusions • Local recurrence rates for patients treated with accelerated partial breast radiation therapy at DMC are comparable to published data. • There was no group or subset found to be at increased risk of recurrence.

Acceptable • • Age > 50 Size < 3 cm Histology ER Margins LVI Nodal status All invasive subtypes and DCIS Positive or negative Not present negative

Not acceptable Outside of a clinical Trial • Node positive • Tumors > 3 cm including DCIS • Extensive LVSI • Neoadjuvant chemo • Age <45 • Multicentric • Microscopically multifocal >3 cm • EIC > 3 cm • Margins + • Nodal surgery- not performed. • BRCA +

Future Directions • Recommendations should be updated annually as new results from prospective randomized trials are released.