nd 2 Semester Chemistry Mrs Schultz schultz 915











- Slides: 16

nd 2 Semester Chemistry Mrs. Schultz schultz 915. pbworks. com

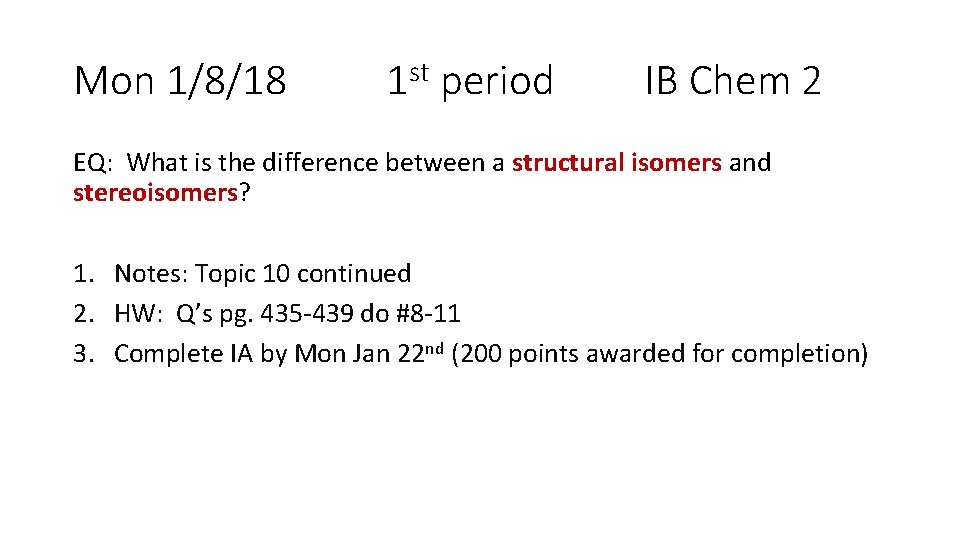
Mon 1/8/18 1 st period IB Chem 2 EQ: What is the difference between a structural isomers and stereoisomers? 1. Notes: Topic 10 continued 2. HW: Q’s pg. 435 -439 do #8 -11 3. Complete IA by Mon Jan 22 nd (200 points awarded for completion)

Mon 1/8/18 2, 4, 5, 7 th period Chem I EQ: Use electron dots to predict the formula for the compound formed between calcium and sulfur. 1. Notes: Ch 7 Ionic bonding 2. HW: Questions pg. 207 (Ch 7 sec. 2)

Mon 1/8/18 3 rd period IB Chem EQ: Define hybridization 1. Notes: Topic 4 continued (begin at part 3 page 9 of the notes) 2. HW: Questions pg. 180 #17 -19

Tues 1/9 2 nd & 4 th period Chemistry I EQ: no question today – sub 1. 2. 3. 4. Due: HW: Q’s pg. 207 #12 -19 CW: WS Ch 7 Reading questions CW: Crossword puzzle Ch 7 & Elemental words puzzle HW: Ch 7 Review Q’s from sec. 7. 1 do #27 -37

Tues 1/9 3 rd period IB Chemistry I IB Group IV project in the media center

Wed 1/10 1 st period IB Group IV project in the media center IB Chem 2

Wed 1/10 5 th & 7 th period Chemistry I EQ: no question today – sub 1. 2. 3. 4. Due: HW: Q’s pg. 207 #12 -19 CW: WS Ch 7 Reading questions CW: Crossword puzzle Ch 7 & Elemental words puzzle HW: Ch 7 Review Q’s from sec. 7. 1 do #27 -37

Wed 1/10 3 rd period IB Chemistry I IB Group IV project in the media center

Thurs 1/11 2 nd & 4 th period Chemistry I *2 nd period 9 th grade assembly at 8: 15 am EQ: Write the formulas for the following ionic compounds: Magnesium fluoride, iron(III) oxide, & aluminum phosphide 1. 2. 3. 4. Due: Ch 7 Review Q’s pg. 214 #27 -37 Notes: Writing ionic compound formulas and names CW: WS Formula practice A HW: Ch 7 Review Questions pg. 214 -215 #38 -46

Thursday 1/11 grade assemblies • 9 th grade - 8: 15 (bring back pack) • 10 th grade - 9: 30 (bring back pack) • 11 th grade - 12: 35 (will return to class) • 12 th grade - 1: 15 (bring back pack)

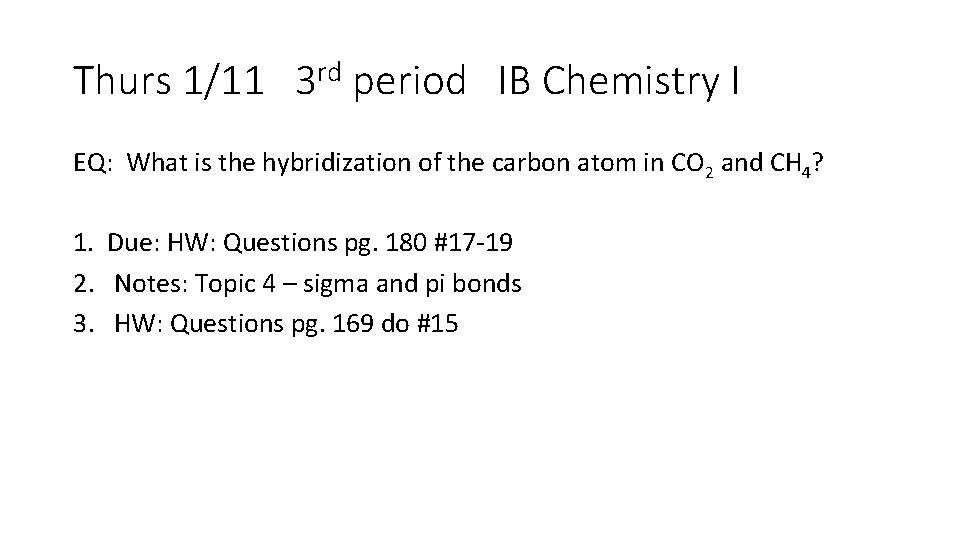
Thurs 1/11 3 rd period IB Chemistry I EQ: What is the hybridization of the carbon atom in CO 2 and CH 4? 1. Due: HW: Questions pg. 180 #17 -19 2. Notes: Topic 4 – sigma and pi bonds 3. HW: Questions pg. 169 do #15

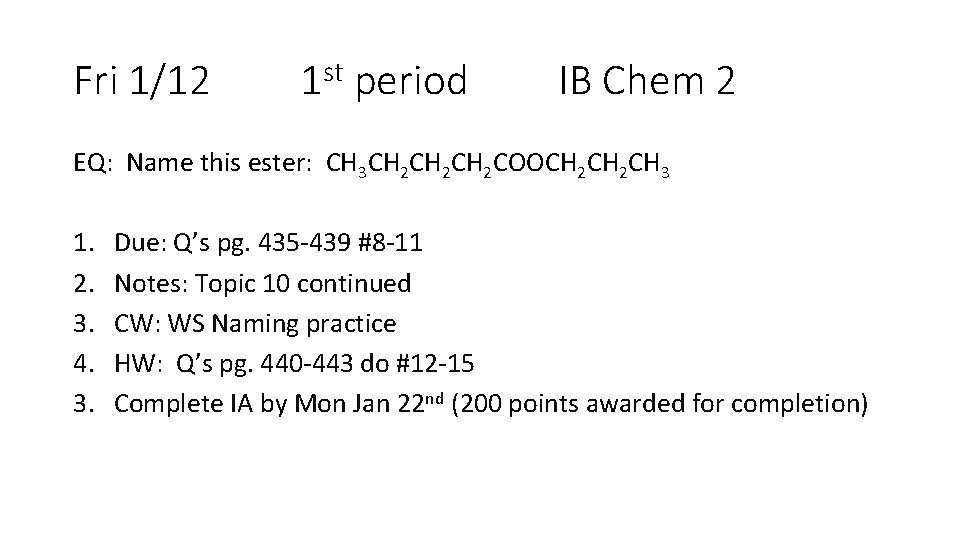
Fri 1/12 1 st period IB Chem 2 EQ: Name this ester: CH 3 CH 2 CH 2 COOCH 2 CH 3 1. 2. 3. 4. 3. Due: Q’s pg. 435 -439 #8 -11 Notes: Topic 10 continued CW: WS Naming practice HW: Q’s pg. 440 -443 do #12 -15 Complete IA by Mon Jan 22 nd (200 points awarded for completion)

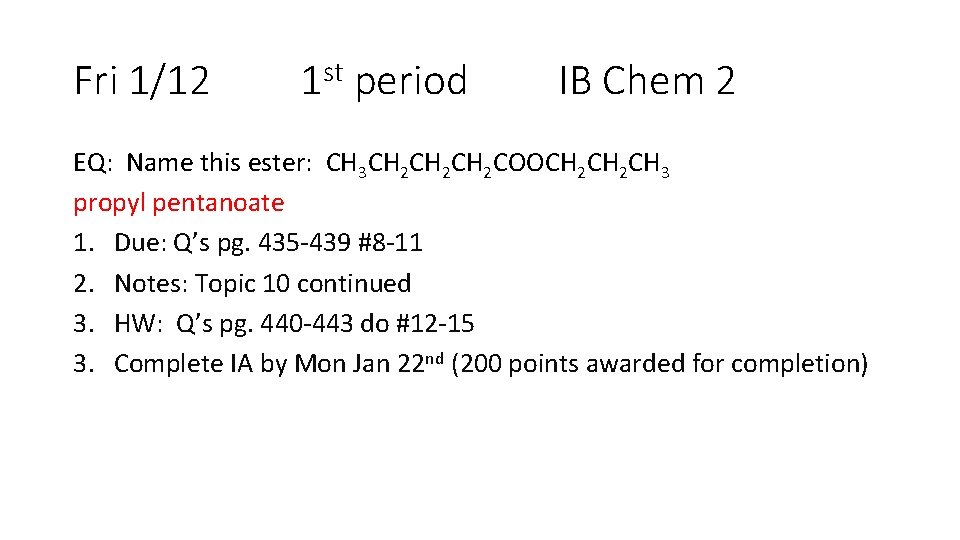
Fri 1/12 1 st period IB Chem 2 EQ: Name this ester: CH 3 CH 2 CH 2 COOCH 2 CH 3 propyl pentanoate 1. Due: Q’s pg. 435 -439 #8 -11 2. Notes: Topic 10 continued 3. HW: Q’s pg. 440 -443 do #12 -15 3. Complete IA by Mon Jan 22 nd (200 points awarded for completion)

Fri 1/12 3 rd period IB Chemistry I EQ: Determine the number of pi bonds and the hybridization of C in a molecule of HCN. 1. Due: HW: Questions pg. 180 #17 -19 and pg. 169 #15 2. Quiz 3. Notes: Topic 4 continued

Fri 1/12 5 th & 7 th period Chemistry I EQ: Write the formulas for the following ionic compounds: Magnesium fluoride, iron(III) oxide, & aluminum phosphide 1. 2. 3. 4. Due: Ch 7 Review Q’s pg. 214 #27 -37 Notes: Writing ionic compound formulas and names CW: WS Formula practice A HW: Ch 7 Review Questions pg. 214 -215 #38 -46