Chemistry class agendas Mrs Schultz Mon 918 Fri









- Slides: 16

Chemistry class agendas Mrs. Schultz Mon 9/18 – Fri 9/22 2017

Mon 9/18 1 st per IB Chem 2 EQ: Determine the concentration of Na. OH(aq) (mol dm-3) when 30. 00 cm 3 of. 200 M HCl(aq) is titrated with Na. OH(aq), the indicator phenolthalein changed from colorless to pink when 60. 00 cm 3 Na. OH(aq) was added. 1. Due: Topic for IA – fill in form and return by Wed. 2. HW: Keep working on WS practice topic 8 Q’s Due this week on Friday 9/22 (Topic 8 test day)

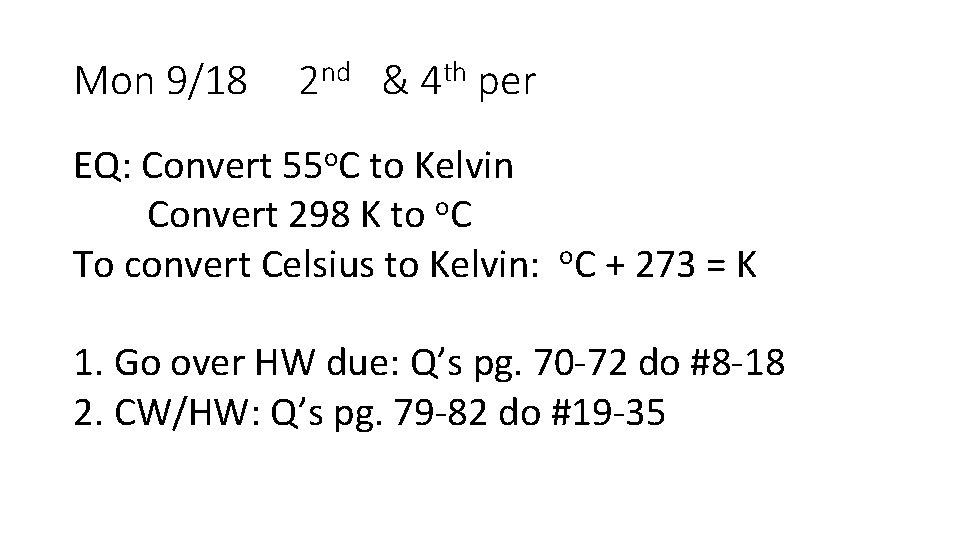
Mon 9/18 2 nd & 4 th per EQ: Convert 55 o. C to Kelvin Convert 298 K to o. C To convert Celsius to Kelvin: o. C + 273 = K 1. Go over HW due: Q’s pg. 70 -72 do #8 -18 2. CW/HW: Q’s pg. 79 -82 do #19 -35

Mon 9/18 3 rd period IB Chem 1 EQ: Describe how you would make 100. 0 cm 3 of a 2. 50 mol dm-3 solution of sodium hydroxide starting with pellets of Na. OH. Back up your explanation with the appropriate calculation. 1. Go over HW: Q’s pg. 45 do #40 -45 2. Notes: Molarity – fill in notes 3. Continue with HW: Exam style Q’s pgs. 51 -53 (due day of topic 1 test – date TBD)

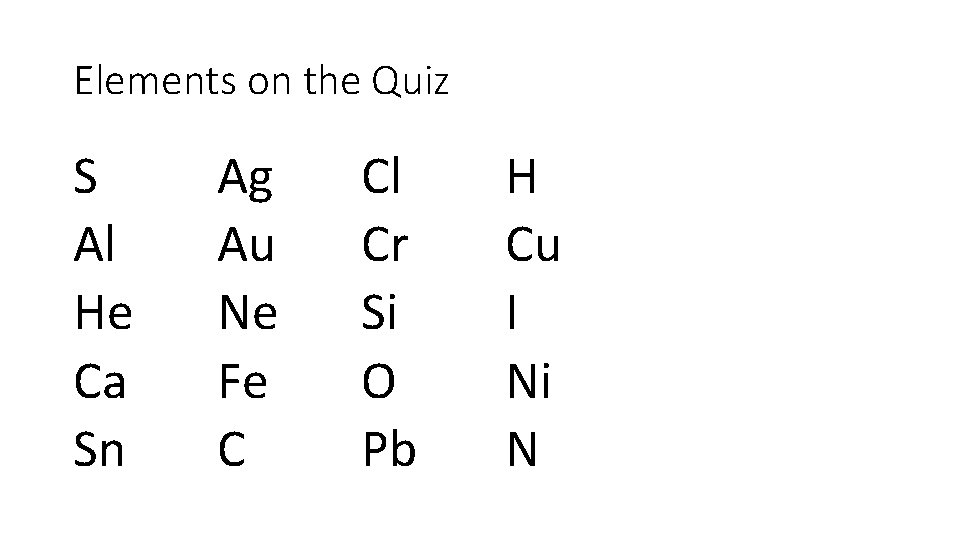
Mon 9/18 5 th & 7 th per Chem PIB-Hon EQ: How many significant digits are in the final answer? Density = mass/volume =300. 0 g/250 m. L = 1. Quiz: 20 elements (S, Ag, Cl, H, Al, Au, Cr, Cu, He, Ne, Si, I, Ca, Fe, O, Ni, Sn, C, Pb, N) 2. Notes: Calculations with scientific notation, SI system, SI conversions 3. HW: Q’s pg. 70 -72 do #8 -18

Elements on the Quiz S Al He Ca Sn Ag Au Ne Fe C Cl Cr Si O Pb H Cu I Ni N

Tues 9/19 2 nd & 4 th per Chem I PIB Honors EQ: How many grams of water are in 35. 76 m. L of water if the density is. 997 g/m. L? 1. Go over HW due: Q’s pg. 79 -82 do #19 -35 2. Lab: Density of two unknown liquids 3. HW: WS density problems

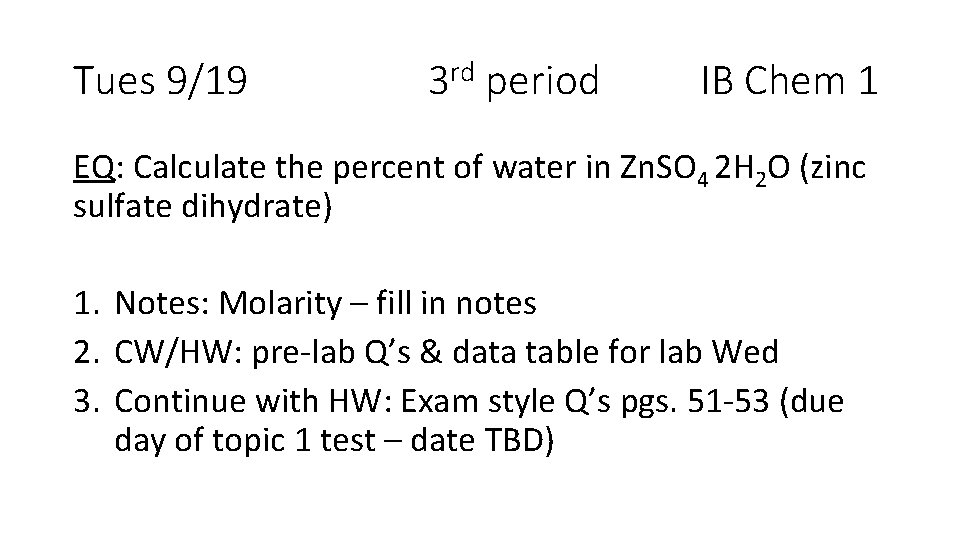
Tues 9/19 3 rd period IB Chem 1 EQ: Calculate the percent of water in Zn. SO 4 2 H 2 O (zinc sulfate dihydrate) 1. Notes: Molarity – fill in notes 2. CW/HW: pre-lab Q’s & data table for lab Wed 3. Continue with HW: Exam style Q’s pgs. 51 -53 (due day of topic 1 test – date TBD)

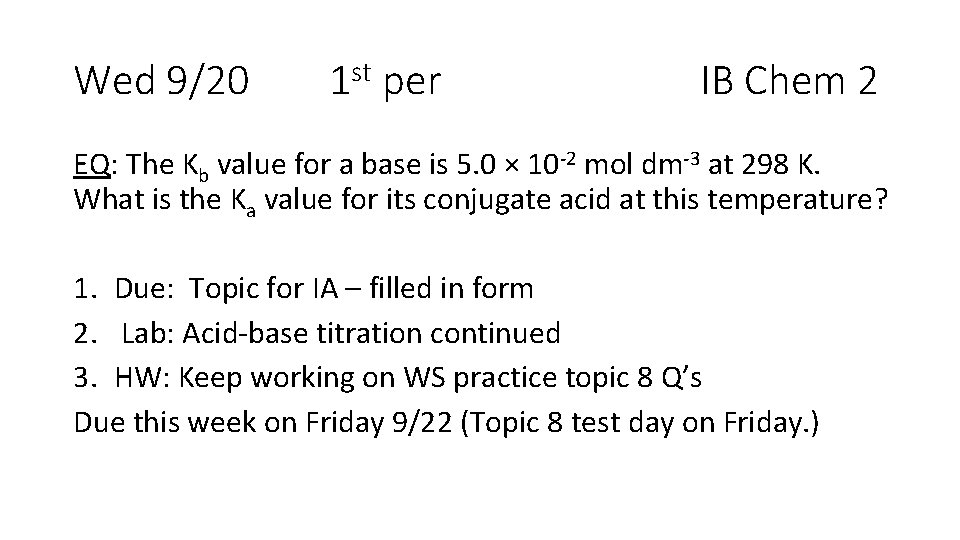
Wed 9/20 1 st per IB Chem 2 EQ: The Kb value for a base is 5. 0 × 10 -2 mol dm-3 at 298 K. What is the Ka value for its conjugate acid at this temperature? 1. Due: Topic for IA – filled in form 2. Lab: Acid-base titration continued 3. HW: Keep working on WS practice topic 8 Q’s Due this week on Friday 9/22 (Topic 8 test day on Friday. )

Wed 9/20 3 rd period IB Chem 1 EQ: 0. 4000 g of hydrated copper sulfate (Cu. SO 4 x. H 2 O) are dissolved in 100. 0 cm 3 of distilled water. 10. 00 cm 3 of this solution are reacted with excess barium chloride solution. The mass of Ba. SO 4 that forms is 3. 739 x 10 -2 g. Calculate the number of moles of Cu. SO 4 in 0. 4000 g of hydrated copper sulfate & determine the value of x. 1. HW due: pre-lab Q’s & data table (for 3 trials. ) 2. Lab: Water in a hydrate 3. Continue with HW: Exam style Q’s pgs. 51 -53 (due day of topic 1 test – date TBD)

Wed 9/20 5 th & 7 th per Chem I PIB Honors EQ: How many grams of water are in 35. 76 m. L of water if the density is. 997 g/m. L? 1. Go over HW due: Q’s pg. 70 -72 do #8 -18 2. Lab: Density of two unknown liquids 3. HW: WS density problems

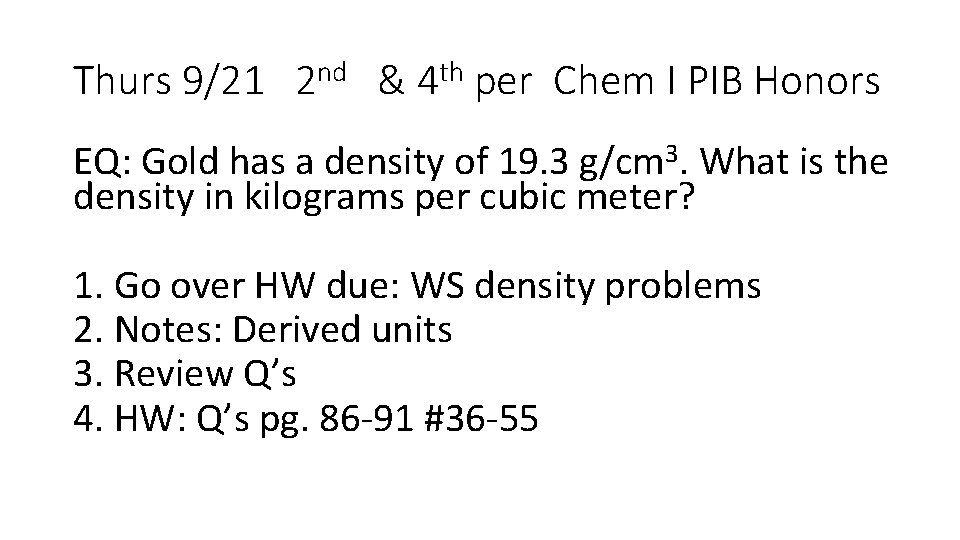
Thurs 9/21 2 nd & 4 th per Chem I PIB Honors EQ: Gold has a density of 19. 3 g/cm 3. What is the density in kilograms per cubic meter? 1. Go over HW due: WS density problems 2. Notes: Derived units 3. Review Q’s 4. HW: Q’s pg. 86 -91 #36 -55

Thurs 9/21 3 rd period IB Chem 1 EQ: no question – today is activity period 1. Continue with Lab: Water in a hydrate on Friday 2. Notes: Fill-in continued… 3. Continue with HW: Exam style Q’s pgs. 51 -53 (due day of topic 1 test – date TBD)

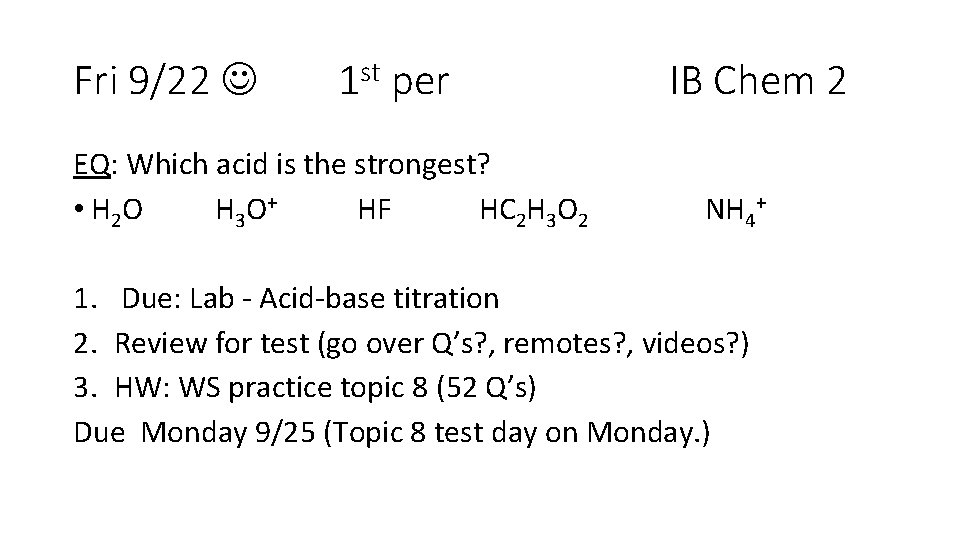
Fri 9/22 1 st per EQ: Which acid is the strongest? • H 2 O H 3 O+ HF HC 2 H 3 O 2 IB Chem 2 NH 4+ 1. Due: Lab - Acid-base titration 2. Review for test (go over Q’s? , remotes? , videos? ) 3. HW: WS practice topic 8 (52 Q’s) Due Monday 9/25 (Topic 8 test day on Monday. )

Fri 9/22 3 rd period IB Chem 1 EQ: no question today – complete lab trials today 1. Finish Lab: Water in a hydrate 2. Continue with HW: Exam style Q’s pgs. 51 -53 (due day of topic 1 test – date TBD) 3. Test on Tuesday 9/26 for topic 1

Fri 9/22 5 th & 7 th per Chem I PIB Honors EQ: Gold has a density of 19. 3 g/cm 3. What is the density in kilograms per cubic meter? 1. Go over HW due: WS density problems 2. Notes: Derived units 3. Review Q’s 4. HW: Q’s pg. 86 -91 #36 -55
 918 saksonya dükü
918 saksonya dükü Decreto 918/2012
Decreto 918/2012 Political agendas examples
Political agendas examples Civicmirror
Civicmirror Civic mirror hidden agendas
Civic mirror hidden agendas Agendas teacch
Agendas teacch Calendar xx
Calendar xx Mon tues wed thurs fri
Mon tues wed thurs fri Mon tues wed thurs fri sat sun
Mon tues wed thurs fri sat sun Fri sat sun
Fri sat sun Wed thr
Wed thr Mon tue wed thur fri sat sun
Mon tue wed thur fri sat sun Mon tue wed thurs fri sat sun
Mon tue wed thurs fri sat sun Mon tues wed
Mon tues wed Mon tue wed
Mon tue wed Wed thurs fri
Wed thurs fri Mon tues wed thurs fri sat sun
Mon tues wed thurs fri sat sun