MOVING skel GEN FORWARD Questions and insights skel


![Allo/Auto/middleground Ref: Harris [internal]; 2013 Combine the ‘best’ of auto with ‘best’ of allo Allo/Auto/middleground Ref: Harris [internal]; 2013 Combine the ‘best’ of auto with ‘best’ of allo](https://slidetodoc.com/presentation_image_h2/fc8471ee9095a2393897f9261fa330ee/image-6.jpg)





![Drivers for commercial success [cont’d] Market driver Weight Notes Reimbursement 6 Current reimbursement level Drivers for commercial success [cont’d] Market driver Weight Notes Reimbursement 6 Current reimbursement level](https://slidetodoc.com/presentation_image_h2/fc8471ee9095a2393897f9261fa330ee/image-14.jpg)

- Slides: 17

MOVING skel. GEN FORWARD Questions and insights skel. GEN meeting University of Minho 16 September 2015 Reg Harris regharris@xtra. co. nz

2

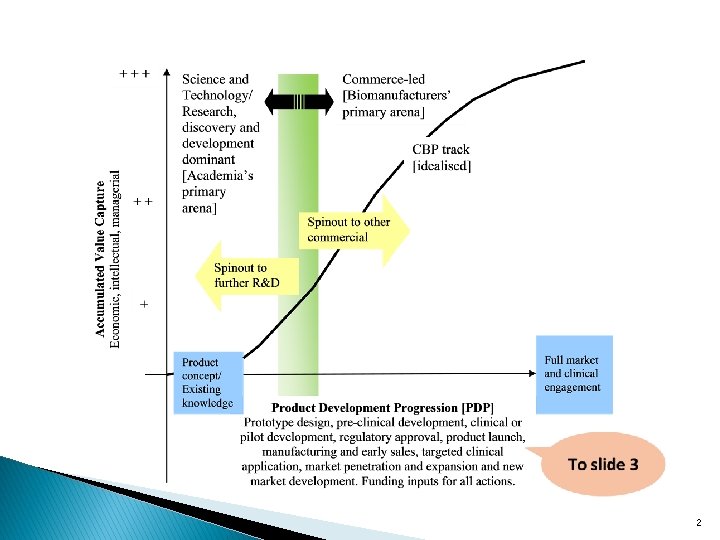
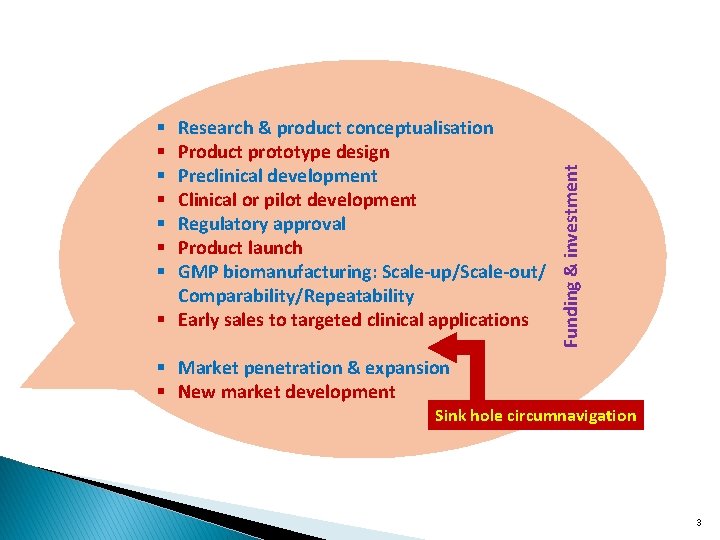
Funding & investment Research & product conceptualisation Product prototype design Preclinical development Clinical or pilot development Regulatory approval Product launch GMP biomanufacturing: Scale-up/Scale-out/ Comparability/Repeatability § Early sales to targeted clinical applications § § § § Market penetration & expansion § New market development Sink hole circumnavigation 3

Academia and industry 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. Industry conversant with the substance and aims of skel. GEN? Academia adequately informed about industry capabilities? Nature and extent of industry involvement? Current status? Strategic fit of skel. GEN products with firm’s existing portfolio? Experience in taking CBPs from lab to market/clinic? Translation of lab-tech to GMP manufacturing process? Focus on manual or automated processes? Control? Clinical translation…moving GMP processes to robust trials? Established distribution channels suited to skel. GEN products? Storage, preservation, packaging & speedy retrieval of CBPs? To slide 5 Business model focus on allogeneic. To orslide autologous? 6 Manufacturing model challenges? 4

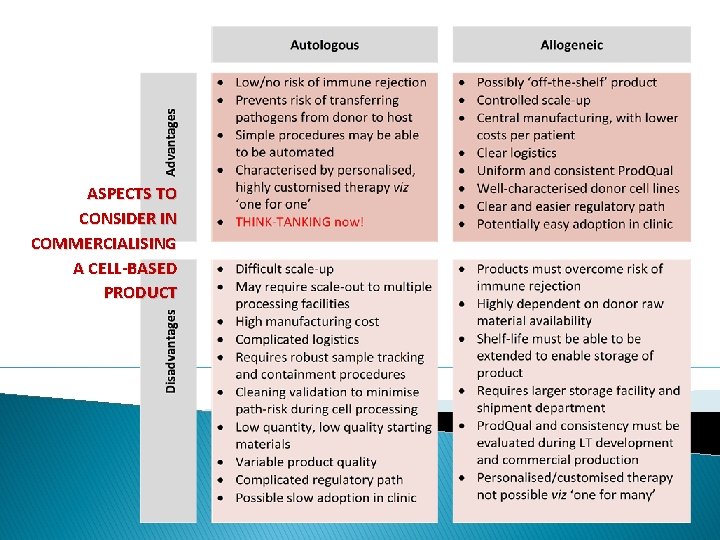
ASPECTS TO CONSIDER IN COMMERCIALISING A CELL-BASED PRODUCT
![AlloAutomiddleground Ref Harris internal 2013 Combine the best of auto with best of allo Allo/Auto/middleground Ref: Harris [internal]; 2013 Combine the ‘best’ of auto with ‘best’ of allo](https://slidetodoc.com/presentation_image_h2/fc8471ee9095a2393897f9261fa330ee/image-6.jpg)
Allo/Auto/middleground Ref: Harris [internal]; 2013 Combine the ‘best’ of auto with ‘best’ of allo • ‘Known and waiting’ patient pools with strong genomic commonality • Patient pools of ‘reasonable’ size • Controlled transfer of therapeutic cells from individual or multiple donors to equal or greater numbers of patients • Dedicated wards with all relevant technology [‘factories-by-proxy’]/close-topatient manufacturing facilities • Means of monitoring and controlling MTMM cells • ‘Immuno-vention’ backup as necessary Model 1 Limited cell expansion Minimal number of population doublings/reduce manufacturing complexity/reduce bio-drift. Model 2 Incremental validation Manufacture MTMM cellular products at multiple sites/release proportional to ‘degree of risk’ ex product release specs in licence/incremental continuous validation after marketing/achieve comparability easily between manufacturing sites [under single-product licence] ex reduced need for characterisation and validation studies. Model 3 Closed or functionally closed automated manufacture MTMM cellular products within a closed and automated process [‘GMP-in-abox’]/achieve comparability easily between manufacturing sites [under single-product licence] ex reduced need for characterisation and validation studies/only the machine would need validation for a chosen indication or protocol and not the sites in which it would be used. Inspired by: Hourd, Ginty, Chandra, Williams; 2013 K N I TH KING N TA Manufacturing models permitting rollout/scale-out of clinically-led autologous cell therapies 6

Clinicians 1. 2. 3. 4. 5. 6. 7. 8. R&D involvement with universities/industry? Discussions about skel. GEN? Projected skel. GEN role e. g. Product conceptualisation, design and specification? Trialling? Counsel in clinical utility? Contribution to evidence base for cost-effectiveness? Ability to interpret and articulate the connections between product development and market/clinical need? To ‘read’ any incongruity within rhythm of a surgical procedure? To slide 8 Innovators? Trail-blazers? Clinical network champions? Degree of satisfaction with extant therapies? Main problems? Looking for more effective ones, including those promising to be efficacious with refractory patients? Willingness and ability to be involved in skel. GEN? 7

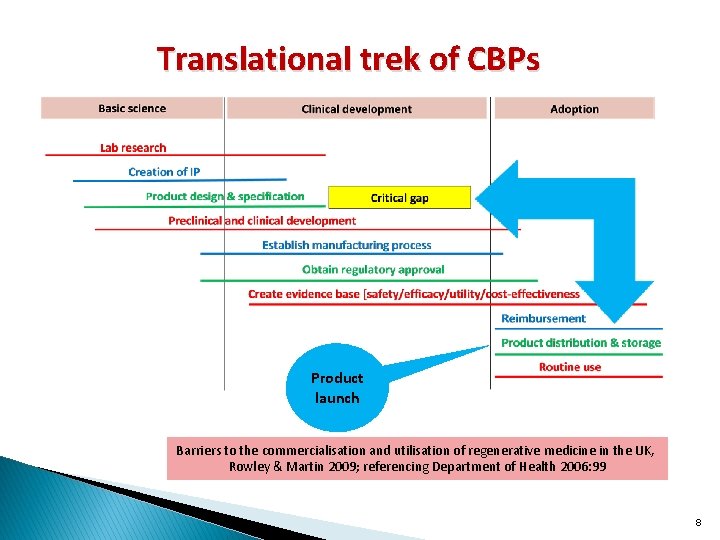
Translational trek of CBPs Product launch Barriers to the commercialisation and utilisation of regenerative medicine in the UK, Rowley & Martin 2009; referencing Department of Health 2006: 99 8

In the clinic 1. 2. 3. 4. 5. 6. Physical products [functioning cells, tissues, organs] or procedures? Marriage of cell therapies: skel. GEN alone or with ‘traditional’? Solutions of high or low complexity? Clinician-friendliness and support staff-friendliness? . . . and generally…. . utility built on existing work practices, routines, resources and infrastructure? Or reasonable compromise? Training: Who does? Where? [Hospital/Po. C, Remote, Dem. Fac] Reimbursement for product and procedure covered? By whom? 9

Challenges and opportunities 1. 2. 3. 4. 5. 6. 7. 8. 9. Priorities for development? [based on perceived markets and access to them, relative ease of navigating regulatory pathways, prospects for patient reimbursement, etc] Critical but inadequately-met needs in the market promising to be met cost-effectively by ‘superior’ skel. GEN products? Differentiated product or race to the bottom? Unique value proposition? Point-of-difference from ‘me too ’ products? Affordability? Adoption? Clinical pull? Positioning of likely competitors? Who are they? Poised to get the jump on skel. GEN? Why and how? skel. GEN moving fast enough to catch main opportunities? Fast, accurate, responsive delivery of solutions to patient? Prospect of a strong patent? Realistic therapeutic targets articulated? To slide 11 10

Therapeutic targeting…. . a skin example Aim: Develop the capability to produce, on demand, clinic-ready A 3 -size[plus] natural [or near-to-natural]/functional skin sheets [epidermal, dermal and subcutaneous layers] to replace, both autologously and allogeneically, severely damaged tissue on the human back with minimal visible seaming with surrounding undamaged native tissue. Minimum expectation: Must be significantly superior clinically, therapeutically, mechanically and economically to extant products, and have good shelf life and storage characteristics. 11

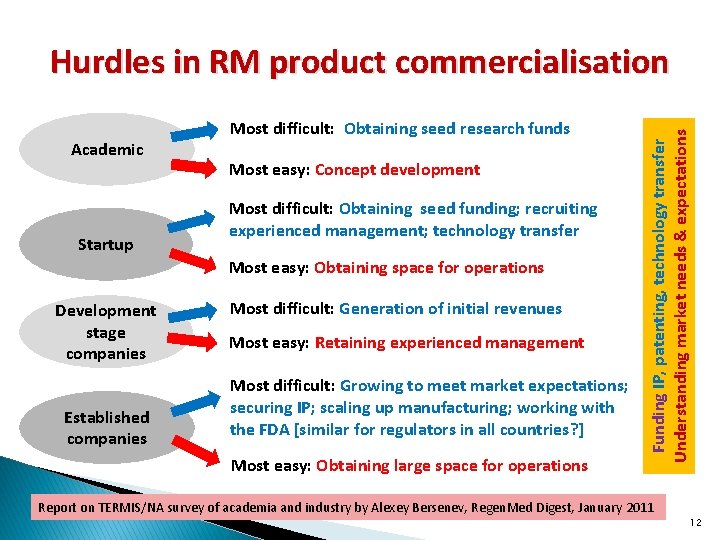
Academic Startup Most difficult: Obtaining seed research funds Most easy: Concept development Most difficult: Obtaining seed funding; recruiting experienced management; technology transfer Most easy: Obtaining space for operations Development stage companies Established companies Most difficult: Generation of initial revenues Most easy: Retaining experienced management Most difficult: Growing to meet market expectations; securing IP; scaling up manufacturing; working with the FDA [similar for regulators in all countries? ] Most easy: Obtaining large space for operations Funding IP, patenting, technology transfer Understanding market needs & expectations Hurdles in RM product commercialisation Report on TERMIS/NA survey of academia and industry by Alexey Bersenev, Regen. Med Digest, January 2011 12

Drivers for commercial success Market driver Weight Notes Clinical efficacy 10 Demonstrated or potential effectiveness of treatment. Animal. Human. Theoretical tools. Market opportunity 9 Patients refractory to current treatments. Number, and rate at which they seek treatment. Intellectual property 8 Freedom to operate. Strong defensible position. Product development efforts/risks 7 Product development efforts required to produce a product for an identified application. Competitive advantage 6 Advantages of the technology applied to the specific clinical indication. Ability to relieve symptoms. Regulatory position 6 Effort and risk associated with achieving regulatory approval. Clinical trial requirements. Sourced and edited from Alfred E. Mann Foundation for Biomedical Engineering. 2006?
![Drivers for commercial success contd Market driver Weight Notes Reimbursement 6 Current reimbursement level Drivers for commercial success [cont’d] Market driver Weight Notes Reimbursement 6 Current reimbursement level](https://slidetodoc.com/presentation_image_h2/fc8471ee9095a2393897f9261fa330ee/image-14.jpg)
Drivers for commercial success [cont’d] Market driver Weight Notes Reimbursement 6 Current reimbursement level and/or reimbursement potential. Physician/Healthcare professional adoption 6 Willingness of physician to adopt the technology assuming ease of use. Access to clinic. Training. Patient motivation/acceptance 6 Willingness of patient to adopt the technology. Competition 5 Positions and strengths of companies operating or looking to operate in the field in focus. Efficacy of their solutions. Points of difference. Strategic fit 4 Complementarity of each indication in relation to others in the company’s product portfolio. Synergy with current products’ distribution. Impact and cost 2 Direct treatment costs. Productivity falloff during gearing up and training.

Questions investors will ask in considering opportunities for commercialising CBPs Is this therapy genuinely innovative, a ‘game changer’, not a ‘me too’? Can it be brought to the clinic in a reasonable timeframe? Is the market for this therapy large enough? Is this solution a single product play or part of a larger platform? Is the intellectual property defensible? Are there strategic partners pinpointed that will share in the development costs? Are there potential licensees or acquirers of this therapy able to manufacture it on a large scale and deliver it through existing marketing channels?

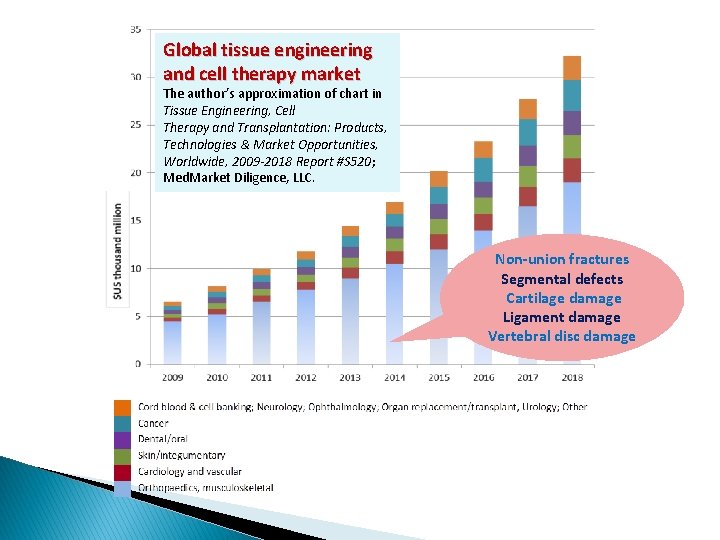
Global tissue engineering and cell therapy market The author’s approximation of chart in Tissue Engineering, Cell Therapy and Transplantation: Products, Technologies & Market Opportunities, Worldwide, 2009 -2018 Report #S 520; Med. Market Diligence, LLC. Non-union fractures Segmental defects Cartilage damage Ligament damage Vertebral disc damage

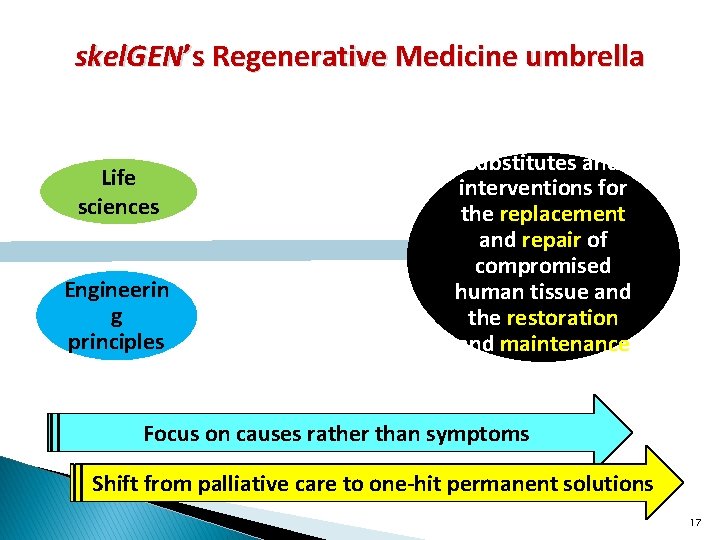
skel. GEN’s Regenerative Medicine umbrella Life sciences Engineerin g principles Biological substitutes and interventions for the replacement and repair of compromised human tissue and the restoration and maintenance of its essential functions Focus on causes rather than symptoms Shift from palliative care to one-hit permanent solutions 17
 Rumus forward rate
Rumus forward rate Forward market adalah
Forward market adalah Dylan williams feedback
Dylan williams feedback Meet the robinson keep moving forward
Meet the robinson keep moving forward Moving dartmouth forward
Moving dartmouth forward Relie check
Relie check Analyzing and using marketing information
Analyzing and using marketing information Presenting insights and findings using written reports
Presenting insights and findings using written reports Insights and understanding ecclesiology
Insights and understanding ecclesiology Queensbridge map
Queensbridge map Relie responsible lending and insights engine
Relie responsible lending and insights engine Relie responsible lending and insights engine
Relie responsible lending and insights engine Relie responsible lending and insights engine
Relie responsible lending and insights engine Relie responsible lending and insights engine
Relie responsible lending and insights engine Data analytics quotes
Data analytics quotes Englanti konjunktiot ja sidesanat
Englanti konjunktiot ja sidesanat Social media consumer insights
Social media consumer insights Maximo asset health insights
Maximo asset health insights