MIT 3 022 Microstructural Evolution in Materials 13


- Slides: 26

MIT 3. 022 Microstructural Evolution in Materials 13: Precipitate Growth Juejun (JJ) Hu hujuejun@mit. edu

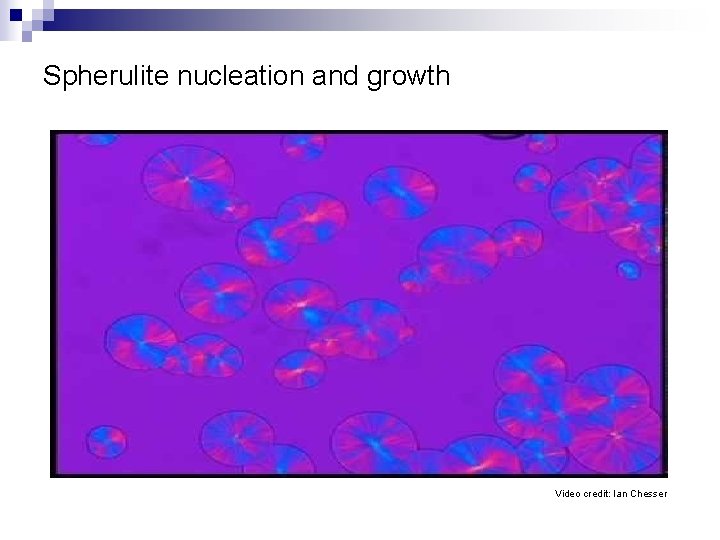
Spherulite nucleation and growth Video credit: Ian Chesser

Classifications of precipitate growth n n Growth from a singlecomponent solution ¨ Faceted precipitates: 2 D nucleation controlled ¨ Precipitates with atomically rough surfaces: interface kinetics controlled Growth from a binary system with miscibility gap ¨ Diffusion controlled Faceted surface

Classifications of precipitate growth n n Growth from a singlecomponent solution ¨ Faceted precipitates: 2 D nucleation controlled ¨ Precipitates with atomically rough surfaces: interface kinetics controlled Non-faceted surface Growth from a binary Temperature system with miscibility gap a ¨ DT b Diffusion controlled 100% A 100% B

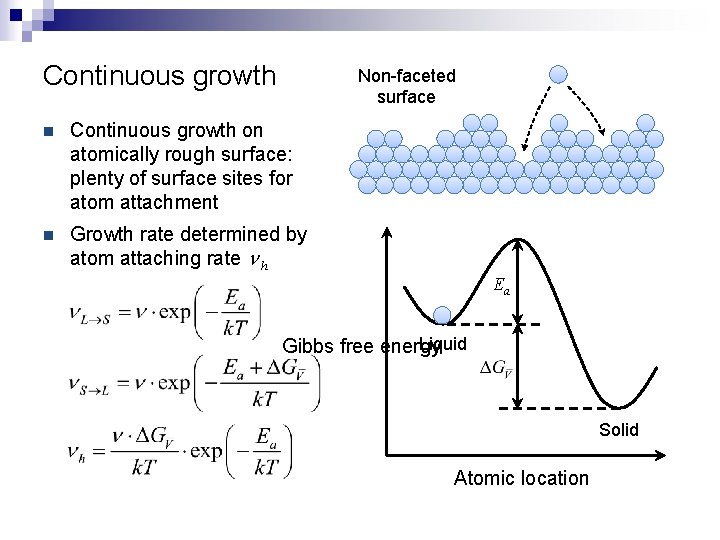
Continuous growth Non-faceted surface n Continuous growth on atomically rough surface: plenty of surface sites for atom attachment n Growth rate determined by atom attaching rate n h Ea Liquid Gibbs free energy Solid Atomic location

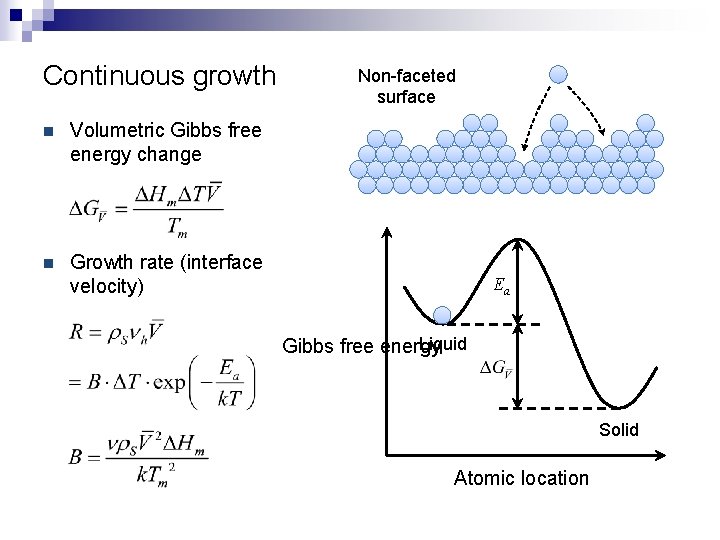
Continuous growth n Volumetric Gibbs free energy change n Growth rate (interface velocity) Non-faceted surface Ea Liquid Gibbs free energy Solid Atomic location

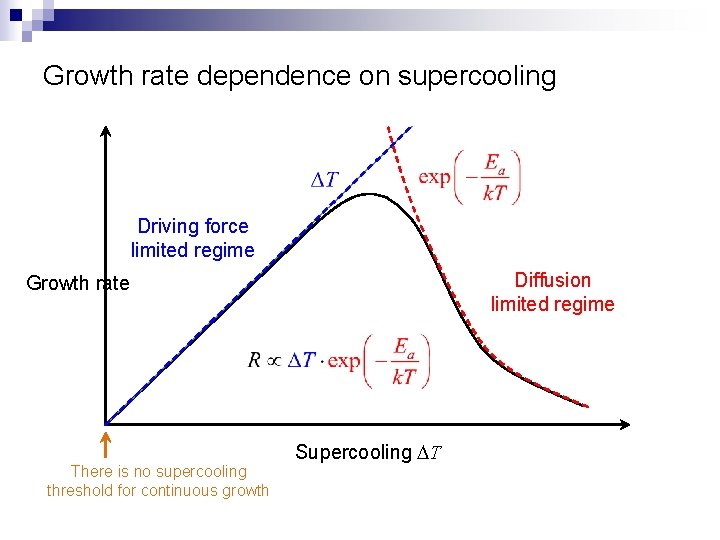
Growth rate dependence on supercooling Driving force limited regime Diffusion limited regime Growth rate There is no supercooling threshold for continuous growth Supercooling DT

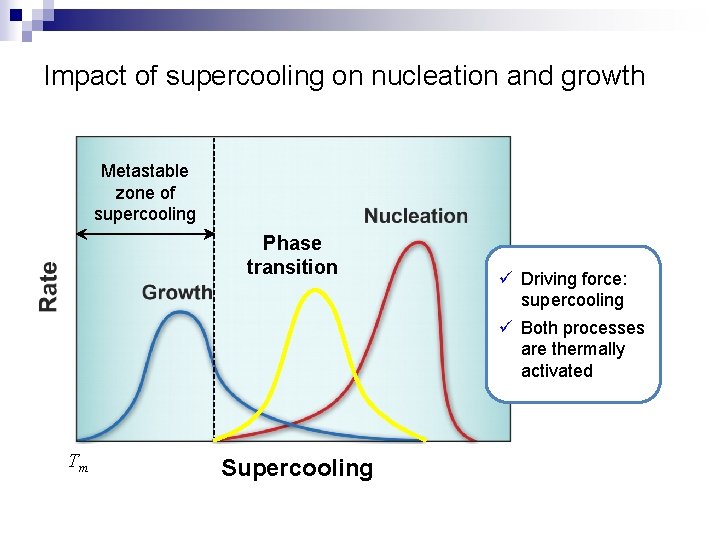
Impact of supercooling on nucleation and growth Metastable zone of supercooling Phase transition Tm Supercooling ü Driving force: supercooling ü Both processes are thermally activated

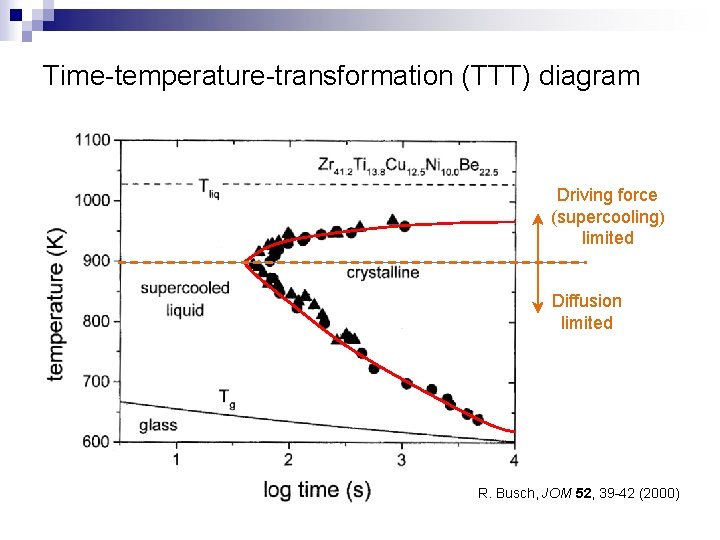
Time-temperature-transformation (TTT) diagram Driving force (supercooling) limited Diffusion limited R. Busch, JOM 52, 39 -42 (2000)

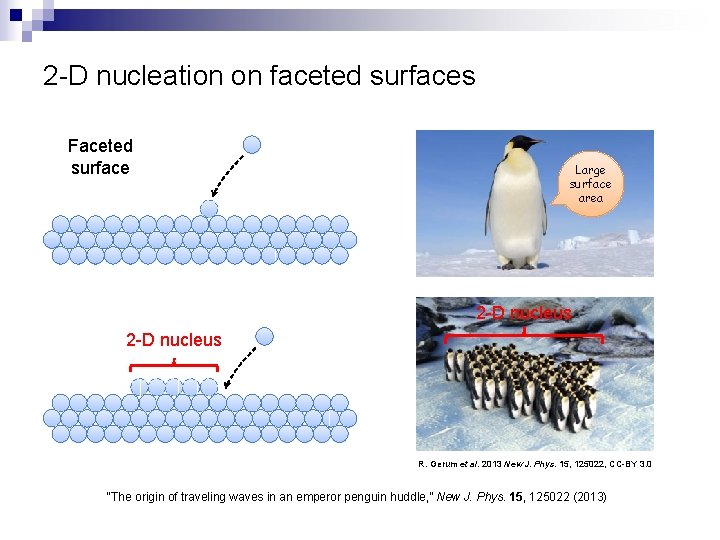
2 -D nucleation on faceted surfaces Faceted surface Large surface area 2 -D nucleus R. Gerum et al. 2013 New J. Phys. 15, 125022, CC-BY 3. 0 “The origin of traveling waves in an emperor penguin huddle, ” New J. Phys. 15, 125022 (2013)

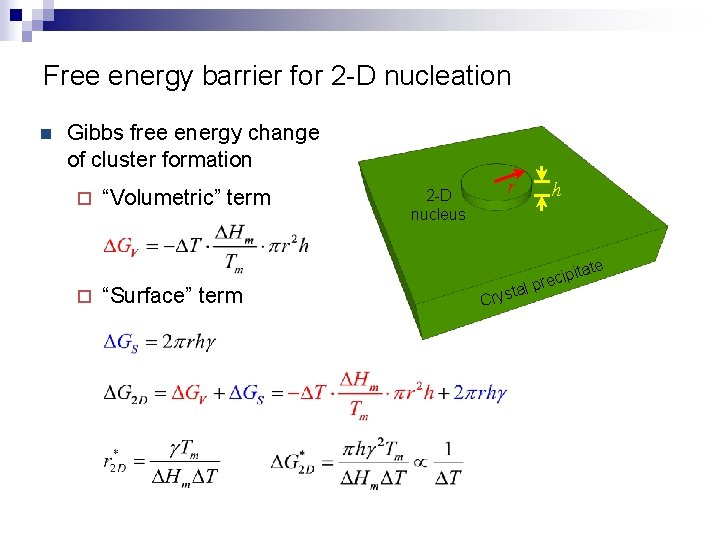
Free energy barrier for 2 -D nucleation n Gibbs free energy change of cluster formation ¨ “Volumetric” term 2 -D nucleus r h te ¨ “Surface” term lp ta Crys ita p i c e r

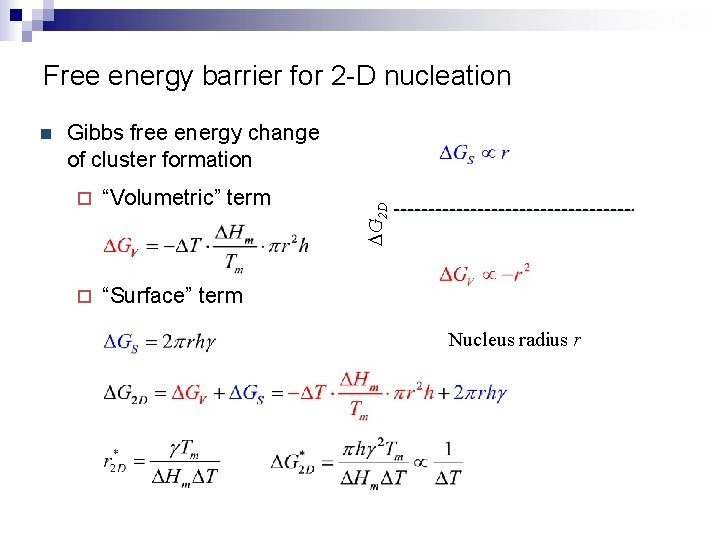
Free energy barrier for 2 -D nucleation Gibbs free energy change of cluster formation ¨ “Volumetric” term ¨ “Surface” term DG 2 D n Nucleus radius r

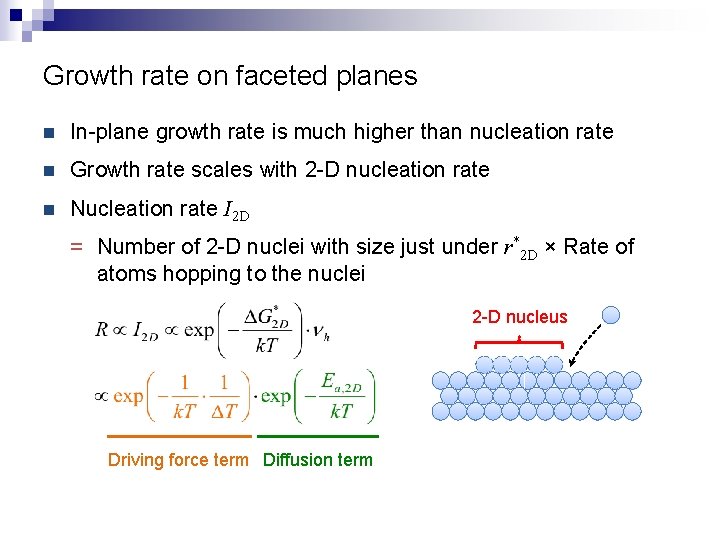
Growth rate on faceted planes n In-plane growth rate is much higher than nucleation rate n Growth rate scales with 2 -D nucleation rate n Nucleation rate I 2 D = Number of 2 -D nuclei with size just under r*2 D × Rate of atoms hopping to the nuclei 2 -D nucleus Driving force term Diffusion term

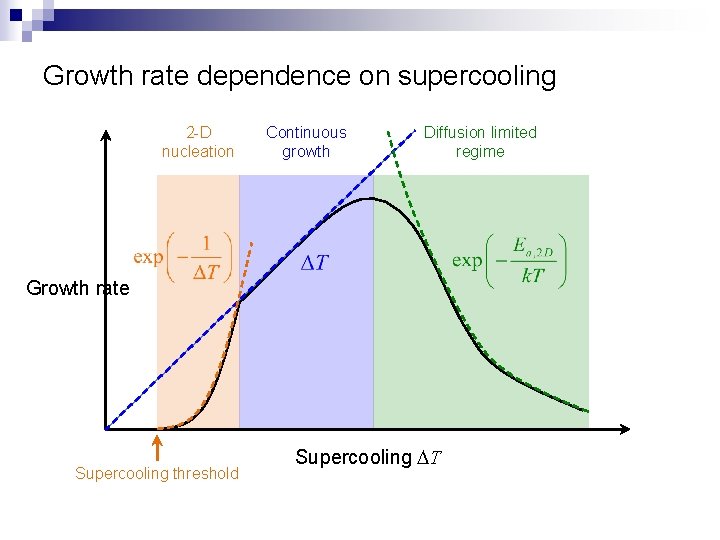
Growth rate dependence on supercooling 2 -D nucleation Continuous growth Diffusion limited regime Growth rate Supercooling threshold Supercooling DT

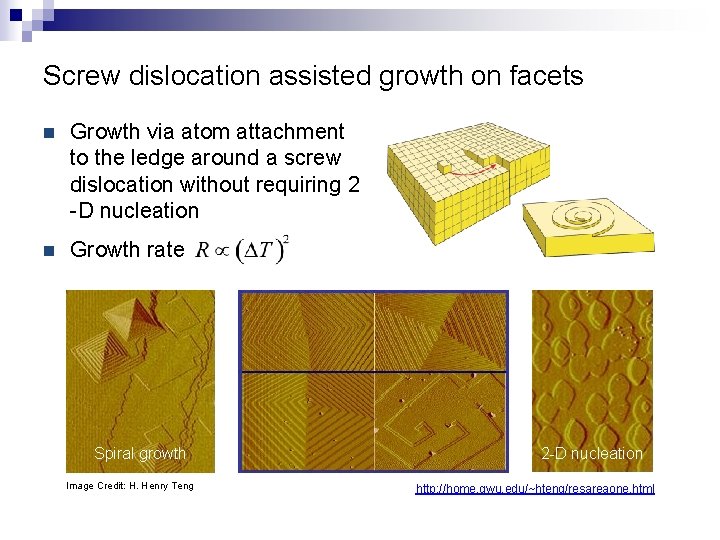
Screw dislocation assisted growth on facets n Growth via atom attachment to the ledge around a screw dislocation without requiring 2 -D nucleation n Growth rate Spiral growth Image Credit: H. Henry Teng 2 -D nucleation http: //home. gwu. edu/~hteng/resareaone. html

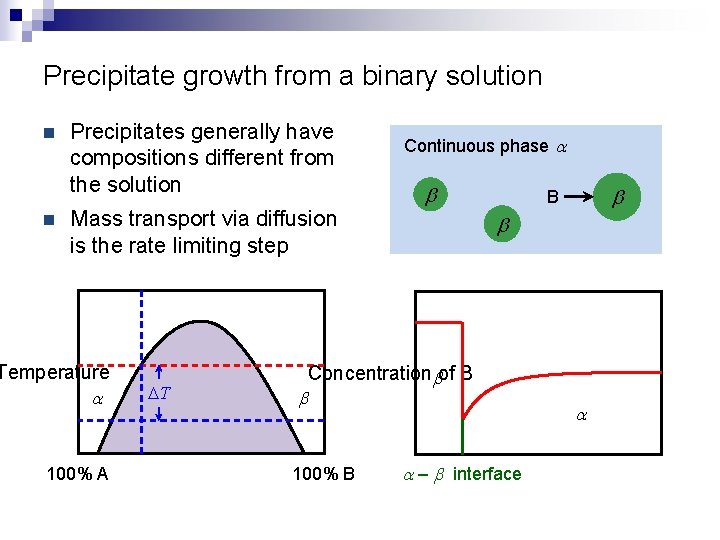
Precipitate growth from a binary solution n n Precipitates generally have compositions different from the solution Mass transport via diffusion is the rate limiting step Temperature a 100% A DT Continuous phase a b b Concentrationbof B b 100% B b B a – b interface a

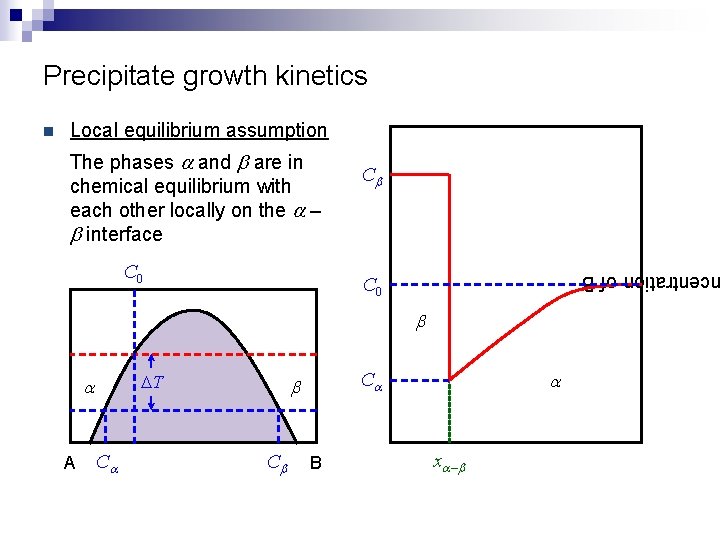
Precipitate growth kinetics Local equilibrium assumption The phases a and b are in chemical equilibrium with each other locally on the a – b interface C 0 Cb centration of B n C 0 b DT a A Ca Cb a Ca b B xa – b

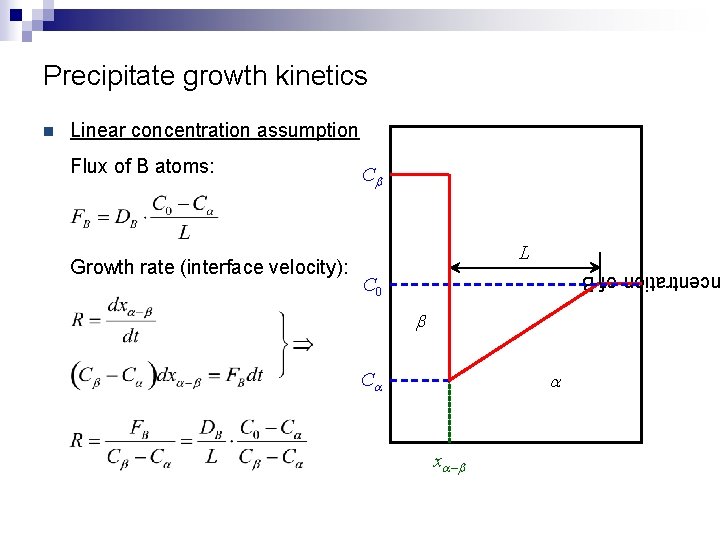
Precipitate growth kinetics Linear concentration assumption Flux of B atoms: Growth rate (interface velocity): Cb L centration of B n C 0 b a Ca xa – b

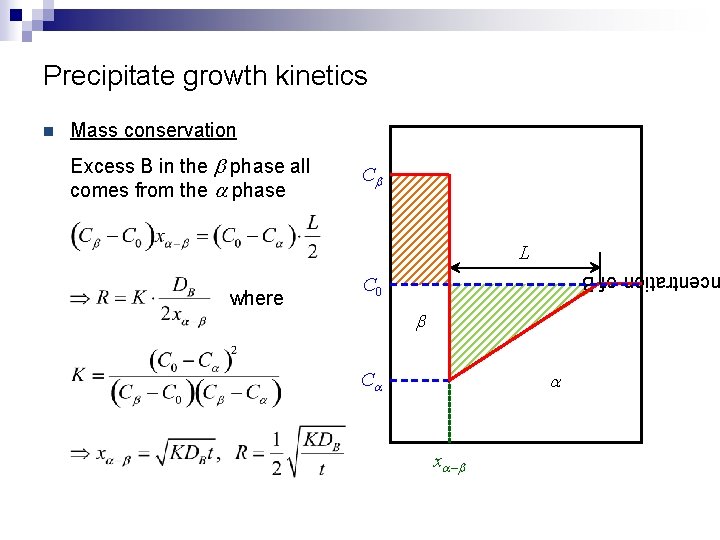
Precipitate growth kinetics Mass conservation Excess B in the b phase all comes from the a phase Cb L where centration of B n C 0 b a Ca xa – b

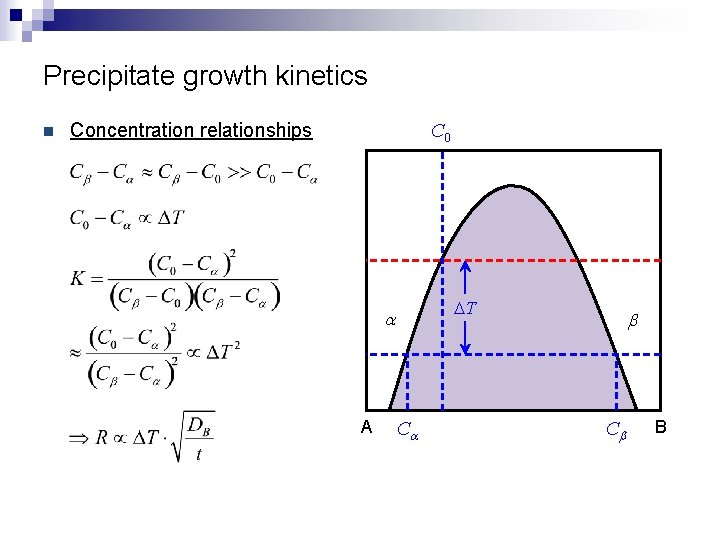
Precipitate growth kinetics n C 0 Concentration relationships DT a A Ca b Cb B

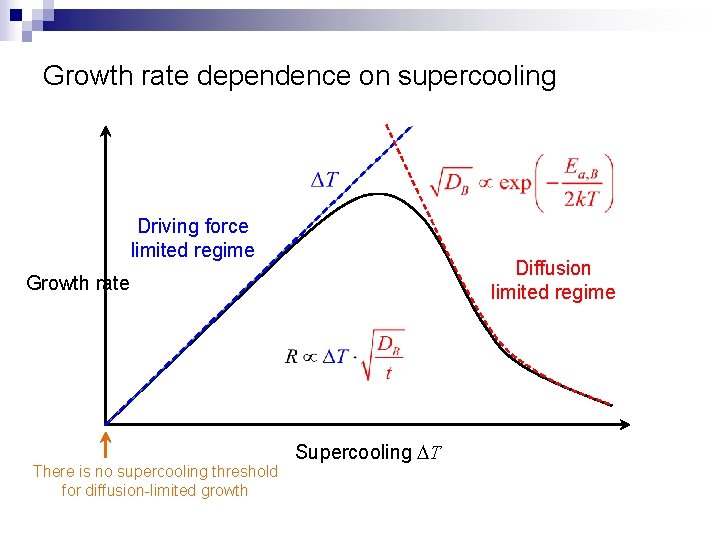
Growth rate dependence on supercooling Driving force limited regime Diffusion limited regime Growth rate There is no supercooling threshold for diffusion-limited growth Supercooling DT

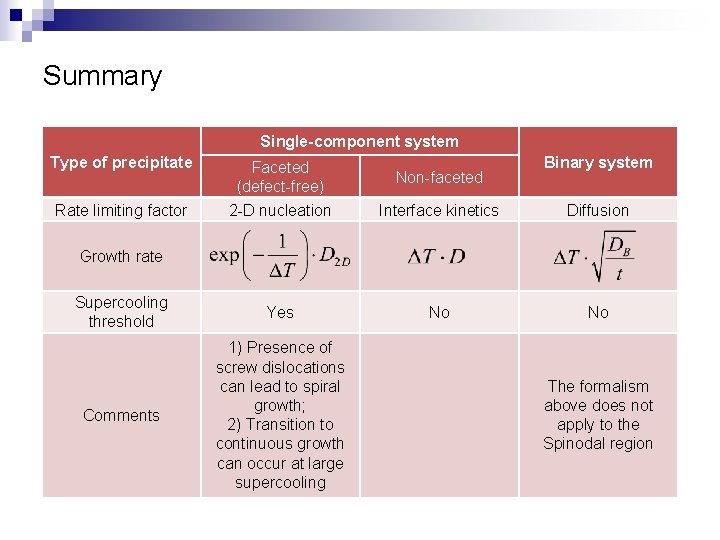
Summary Single-component system Type of precipitate Binary system Faceted (defect-free) 2 -D nucleation Interface kinetics Diffusion Supercooling threshold Yes No No Comments 1) Presence of screw dislocations can lead to spiral growth; 2) Transition to continuous growth can occur at large supercooling Rate limiting factor Non-faceted Growth rate The formalism above does not apply to the Spinodal region

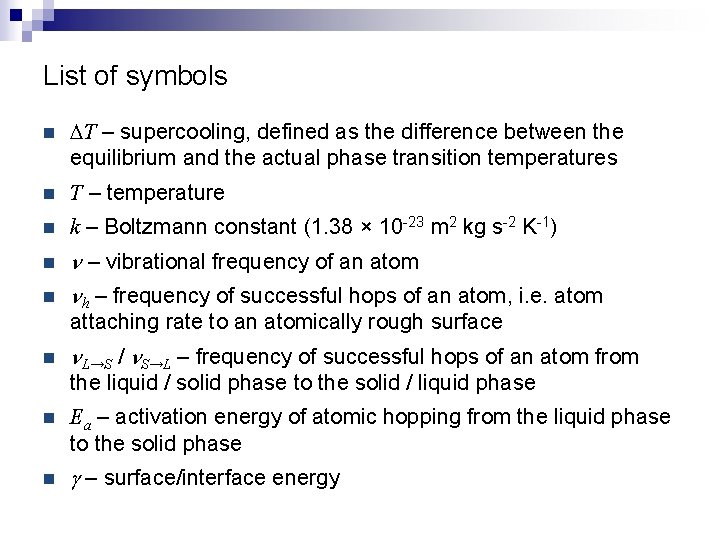
List of symbols n DT – supercooling, defined as the difference between the equilibrium and the actual phase transition temperatures n T – temperature n k – Boltzmann constant (1. 38 × 10 -23 m 2 kg s-2 K-1) n n – vibrational frequency of an atom nh – frequency of successful hops of an atom, i. e. atom n attaching rate to an atomically rough surface n n. L→S / n. S→L – frequency of successful hops of an atom from the liquid / solid phase to the solid / liquid phase n Ea – activation energy of atomic hopping from the liquid phase to the solid phase n g – surface/interface energy

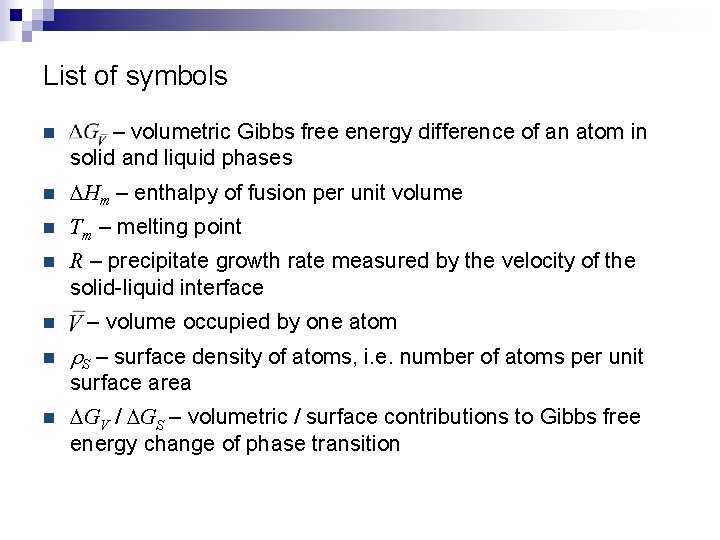
List of symbols n – volumetric Gibbs free energy difference of an atom in solid and liquid phases n DHm – enthalpy of fusion per unit volume n Tm – melting point n R – precipitate growth rate measured by the velocity of the solid-liquid interface n n – volume occupied by one atom r. S – surface density of atoms, i. e. number of atoms per unit surface area n DGV / DGS – volumetric / surface contributions to Gibbs free energy change of phase transition

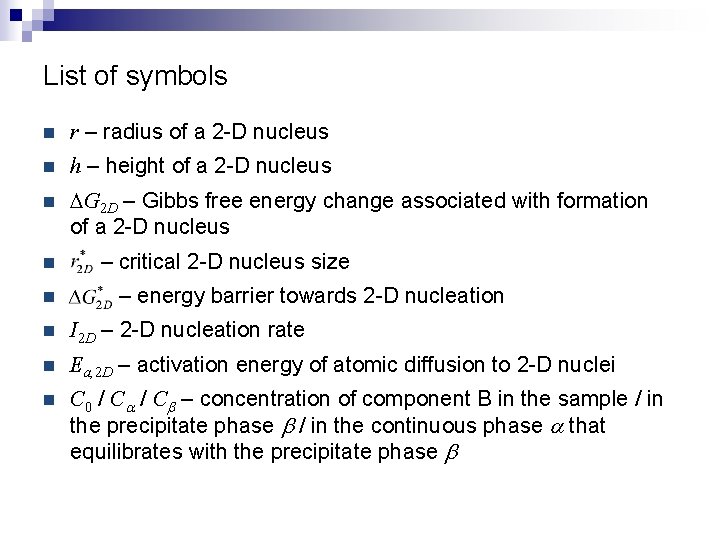
List of symbols n r – radius of a 2 -D nucleus n h – height of a 2 -D nucleus n DG 2 D – Gibbs free energy change associated with formation of a 2 -D nucleus n n – critical 2 -D nucleus size – energy barrier towards 2 -D nucleation n I 2 D – 2 -D nucleation rate n Ea, 2 D – activation energy of atomic diffusion to 2 -D nuclei n C 0 / Ca / Cb – concentration of component B in the sample / in the precipitate phase b / in the continuous phase a that equilibrates with the precipitate phase b

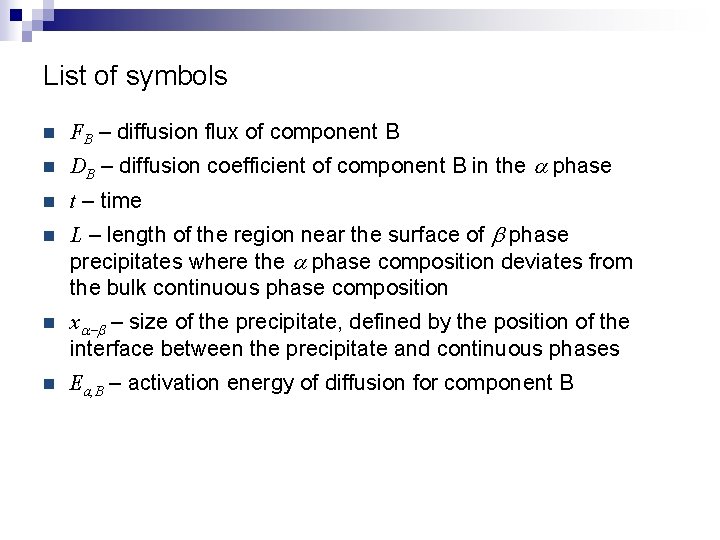
List of symbols n FB – diffusion flux of component B n DB – diffusion coefficient of component B in the a phase n t – time n L – length of the region near the surface of b phase precipitates where the a phase composition deviates from the bulk continuous phase composition n xa-b – size of the precipitate, defined by the position of the interface between the precipitate and continuous phases n Ea, B – activation energy of diffusion for component B