Mine Spoil Weathering and TDS Dynamics W Lee



















- Slides: 36

Mine Spoil Weathering and TDS Dynamics W. Lee Daniels, Zenah Orndorff and Carl Zipper Dept. of Crop and Soil Environ. Sciences

Historically, for active surface mines, we have focused our premining analytics on (1) which materials need to be treated/isolated to prevent AMD and (2) which materials are optimal revegetation substrates However, we now need to consider (3) what TDS components will each release?

Where’s TDS come from? • Acid-base reactions; sulfide oxidation and carbonate neutralization reactions. • Background carbonation reactions in non -sulfidic materials. • Hydrolysis of primary mineral grains. • Entrained Cl and SO 4 in rocks (minor). • Other minor weathering reactions like K release from micas, etc.

All these reduced gray spoils are just waiting to weather!

Oxidized, p. H 5. 5 overburden over reduced p. H 8. 0 carbonate (2%) containing overburden at depth.

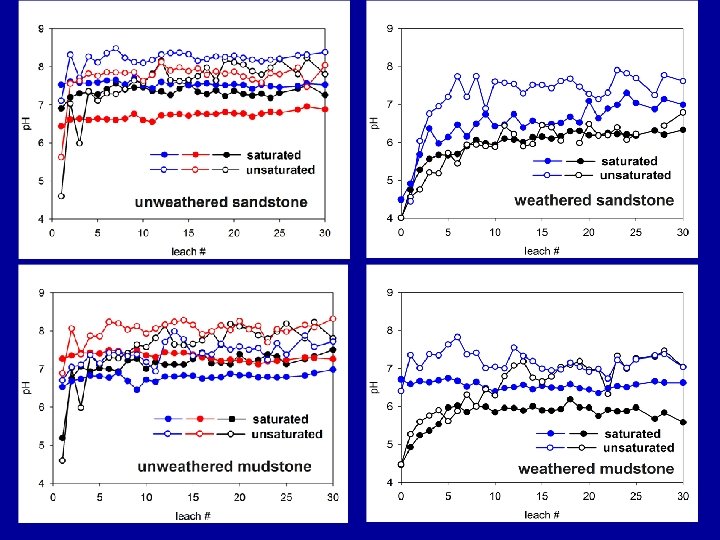
Sample Collection • Mine spoils collected from SW Virginia and Eastern Kentucky (> 25 to date). • In many cases spoils are “paired” by site to compare weathered/oxidized materials vs. unweathered/reduced. • Composite samples (per shot) collected from drill cores from two sites in SW VA.

Minimally weathered/reduced Well-weathered/oxidized Mixed materials diverse spoil types represented: sandstones siltstones mudstones different degrees of weathering

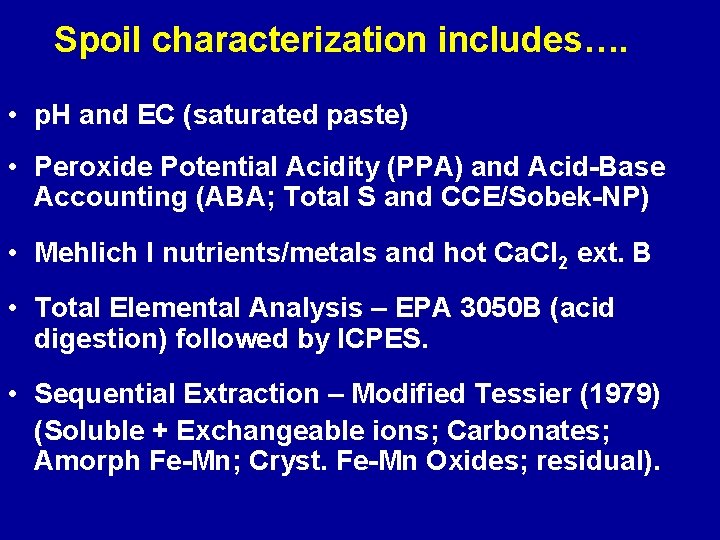
Spoil characterization includes…. • p. H and EC (saturated paste) • Peroxide Potential Acidity (PPA) and Acid-Base Accounting (ABA; Total S and CCE/Sobek-NP) • Mehlich I nutrients/metals and hot Ca. Cl 2 ext. B • Total Elemental Analysis – EPA 3050 B (acid digestion) followed by ICPES. • Sequential Extraction – Modified Tessier (1979) (Soluble + Exchangeable ions; Carbonates; Amorph Fe-Mn; Cryst. Fe-Mn Oxides; residual).

COLUMN LEACHING Capped with ~5 cm sand • samples air-dried and crushed to pass a 1. 25 -cm sieve • Inside diameter = 7. 4 cm • Length/height = 45+ cm • Inside bottom of column: ~5 cm sand layer above Whatman #1 filter above 0. 1 mm mesh (glued to endcap) • 1 cm PVC pipe nipple and Tygon tubing for drainage • Sample volume ~1200 cm 3

• Each sample run in triplicate under saturated and/or unsaturated conditions (3 to 6 columns per sample) • Typically run for minimum of 20 weeks (40 cycles)

Column Leaching Simulated acid rain (p. H ~4. 8) applied 2 x/week (Mon/Th. ) Each rainfall event = 100 ml (~2. 5 cm – 1 inch) Samples (100 ml) collected after 24 hrs (Tue/Fri). Samples analyzed for: p. H, EC, Ca, Fe, Mn, HCO 3 - , S, Cl, Se, etc…. Several other parameters (i. e. TDS by wt. , trace metals) may be evaluated.


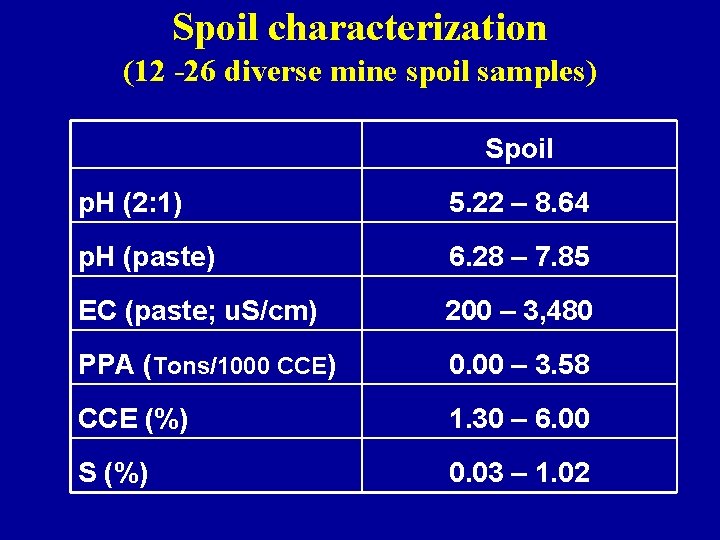
Spoil characterization (12 -26 diverse mine spoil samples) Spoil p. H (2: 1) 5. 22 – 8. 64 p. H (paste) 6. 28 – 7. 85 EC (paste; u. S/cm) 200 – 3, 480 PPA (Tons/1000 CCE) 0. 00 – 3. 58 CCE (%) 1. 30 – 6. 00 S (%) 0. 03 – 1. 02


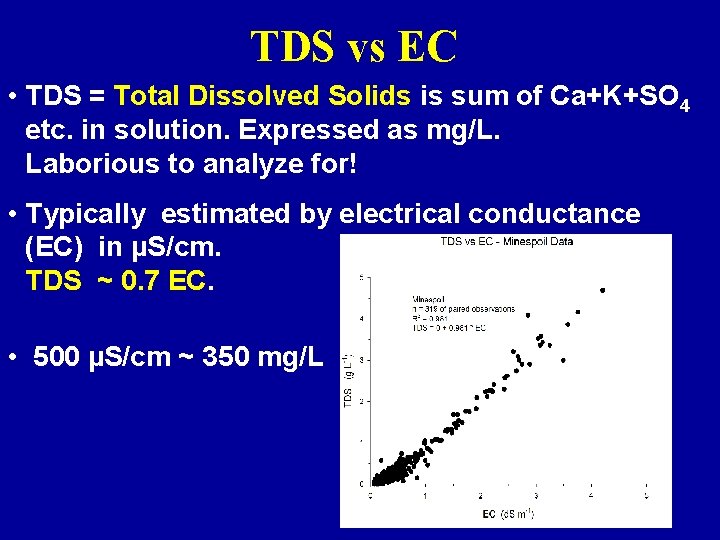
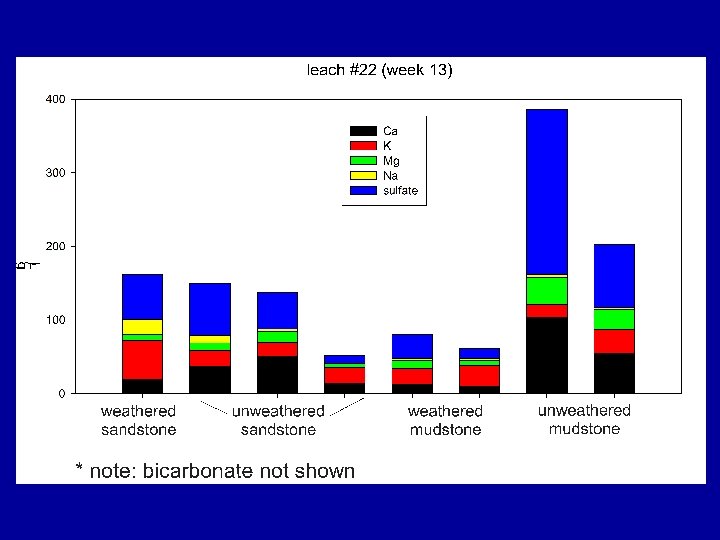
TDS vs EC • TDS = Total Dissolved Solids is sum of Ca+K+SO 4 etc. in solution. Expressed as mg/L. Laborious to analyze for! • Typically estimated by electrical conductance (EC) in µS/cm. TDS ~ 0. 7 EC. • 500 µS/cm ~ 350 mg/L

unsaturated


unsaturated


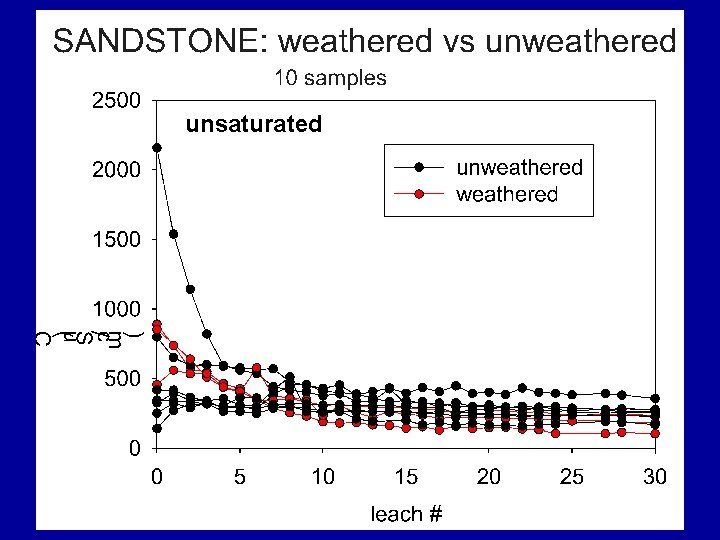
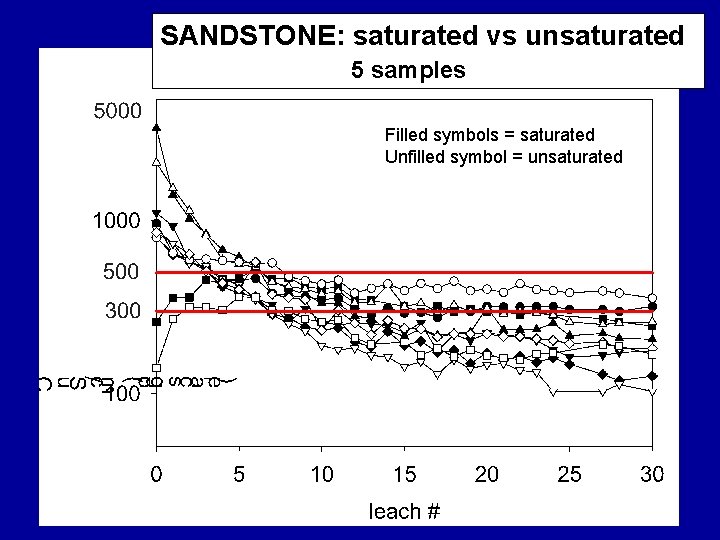
SANDSTONE: saturated vs unsaturated 5 samples Filled symbols = saturated Unfilled symbol = unsaturated

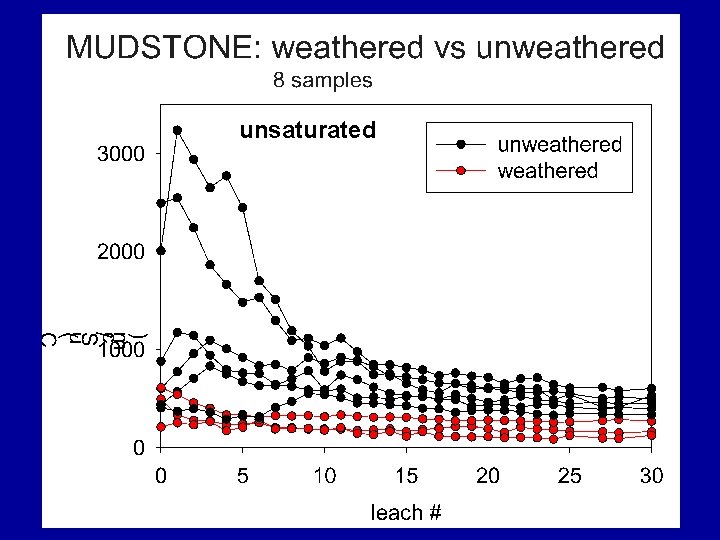
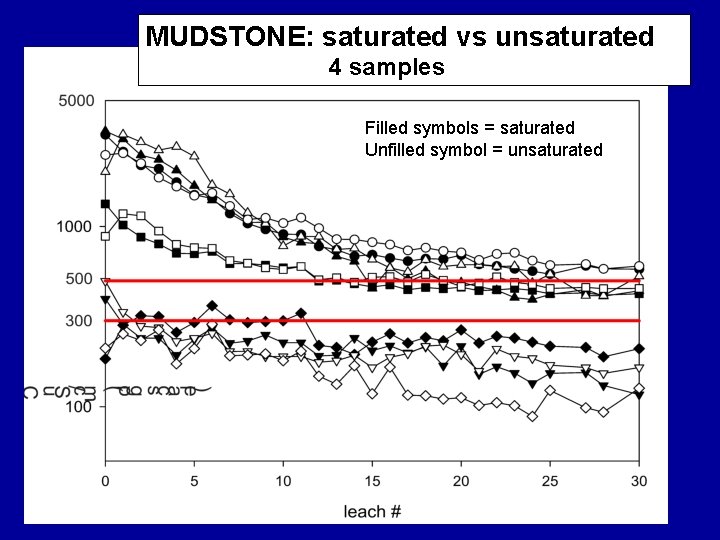
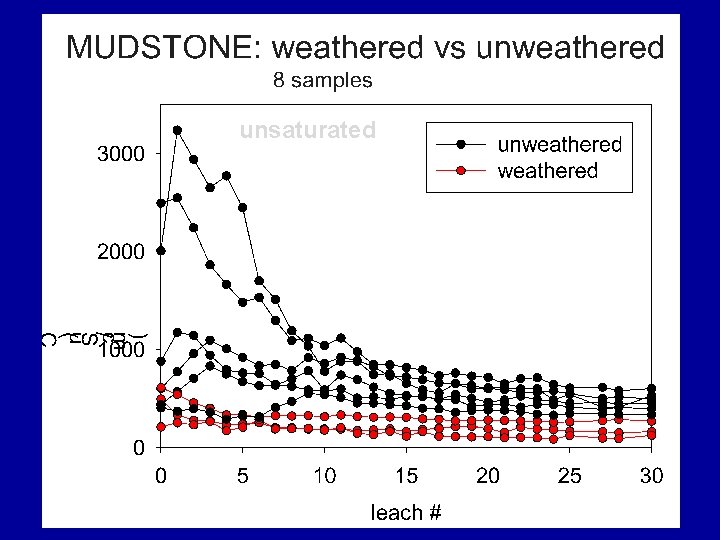
MUDSTONE: saturated vs unsaturated 4 samples Filled symbols = saturated Unfilled symbol = unsaturated


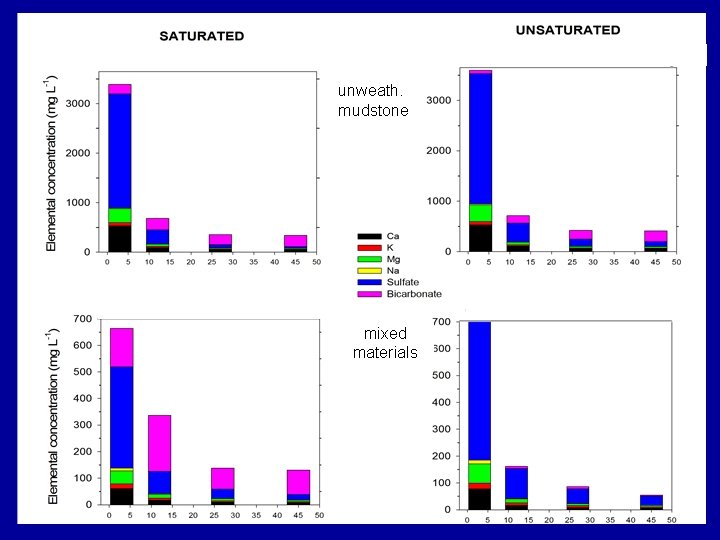
unweath. mudstone mixed materials

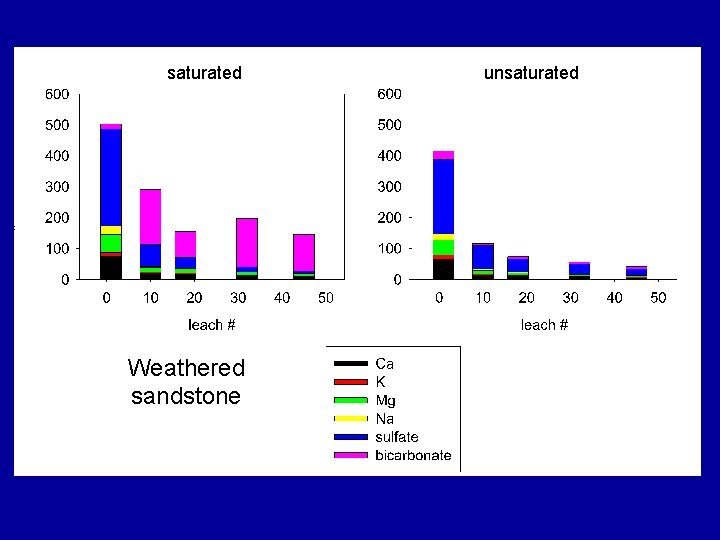
saturated Weathered sandstone unsaturated

unsaturated

Summary Findings Most samples eluted considerable levels of TDS over their initial leaching cycles with EC usually > 500 u. S/cm; some were lower. For all materials, after first pore volumes (7 to 9 cycles) leach, TDS elution drops rapidly. Samples containing significant reactive sulfides were most likely to elute high TDS levels for the duration of the study, regardless of their leachate p. H values.

Summary Findings Overall, TDS mass was dominated by six elements/compounds: Ca, K, Mg, Na, SO 4 and HCO 3, . TDS was commonly dominated by… • sulfate under unsaturated conditions • sulfate and bicarbonate under saturated conditions.

Summary Findings • Many spoils generate drainage with moderate to high p. H and high TDS. • TDS evolution will be directly related to the source strata and extent of historic weathering and oxidation. “Brown is better!” • Similar to our historical approach to acidbase-accounting, we need to develop better predictive tools for TDS release.

How can we do it? • Assume all pyritic-S will leach as sulfate over time. Use weak H 2 O 2 to predict S reactivity? • Measure CCE and assume all will leach as Ca + bicarbonate over time. But how quickly? • Use saturated paste EC to predict “first flush peaks” and ABA parameters + other analytics via regression to estimate longer term release. • Predicting the peak of TDS and the shape of the long-term release slope will take some work!

unsaturated

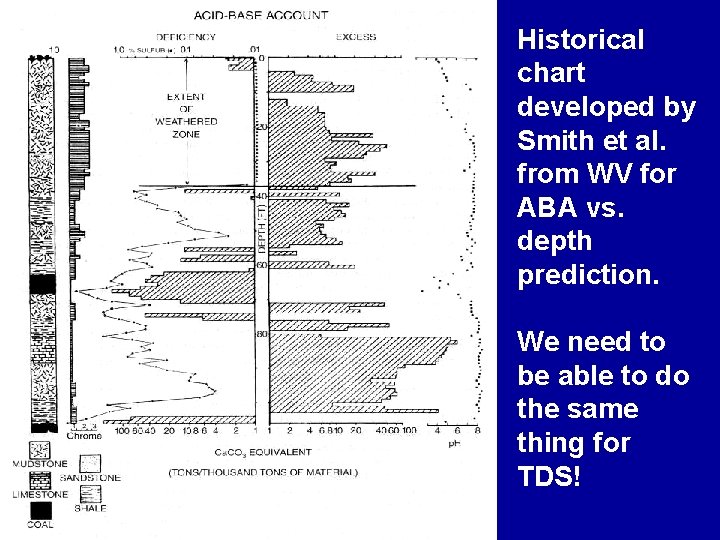
Historical chart developed by Smith et al. from WV for ABA vs. depth prediction. We need to be able to do the same thing for TDS!

Spoil Handling and Placement • Identify “hot TDS” materials and isolate them in similar fashion to acid forming strata. • Avoid durable rock fills where hard/gray unoxidized rocks (with even moderate TDS potential) are placed into direct percolation/leaching zones. • Don’t mix acid-forming and net alkaline materials in fills to intentionally meet ABA. This is a TDS engine. • Consider alternative fill designs where surface lifts are compacted to minimize infiltration.

Regional TDS Prediction Study I. Obtain a representative regional sample set of mine spoils and associated weathered overburden from the central Appalachian region with varying potentials for TDS release. II. Fully analyze these spoil materials via a wide range of laboratory analytical procedures for their potential to release important TDS components upon leaching and weathering. III. Characterize the TDS elution behavior of selected mine spoil materials via column leaching analyses for TDS and component ions of interest.

Regional TDS Prediction Study IV. Determine whether predictive relationships exist between the various lab procedures employed to estimate TDS release potential and the actual TDS flux behavior observed from the leaching columns. V. Investigate the effect of leaching scale (columns vs. tanks vs. fills) on the quantity and temporal nature of TDS release from selected mine spoils. VI. Relate laboratory TDS predictors to actual field data sets for coal mining operations dominated by the spoils tested in this study.

Oxidized, p. H 5. 5 overburden over reduced carbonate (2%) containing overburden at depth.

Acknowledgments Current support provided by industry cooperators and Powell River Project. Thanks to OSM for support of original 2007 to 2009 leaching study. Thanks to Red River Coal, Alpha Natural Resources, TECO and other industry cooperators for assistance in sample collection.
 Porphyria's lover annotated
Porphyria's lover annotated Types of chemical weathering
Types of chemical weathering Agents of physical weathering
Agents of physical weathering Pedalfers
Pedalfers What cause physical weathering
What cause physical weathering Food items that can get spoilt in 2-3 days
Food items that can get spoilt in 2-3 days Triangular spoil bank
Triangular spoil bank Spoil wildlife
Spoil wildlife Https://bit.ly/frequent_flier_door_swing
Https://bit.ly/frequent_flier_door_swing Spoil my appetite
Spoil my appetite Examples of little foxes that spoil the vine
Examples of little foxes that spoil the vine Examples of little foxes that spoil the vine
Examples of little foxes that spoil the vine Turbidity apes
Turbidity apes The first tds equation is
The first tds equation is Tds in software testing
Tds in software testing Formula entalpia
Formula entalpia Tds on conference hall rent
Tds on conference hall rent What is tds relation
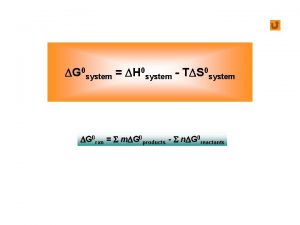
What is tds relation Dg=dh-tds
Dg=dh-tds Dg=-rtlnk
Dg=-rtlnk Dg=dh-tds
Dg=dh-tds Tds 210
Tds 210 Easy tds software
Easy tds software Unicorn vs tds
Unicorn vs tds Tds penumbra
Tds penumbra Easy tds software
Easy tds software Ibuprofen 400mg tds
Ibuprofen 400mg tds Tds
Tds Wouter slegers
Wouter slegers Express tds
Express tds Rocketeer tds
Rocketeer tds Python tds
Python tds Mine reclamation before and after
Mine reclamation before and after He is mine and i am his
He is mine and i am his I acknowledge mine answers
I acknowledge mine answers Compare and contrast mechanical and chemical weathering
Compare and contrast mechanical and chemical weathering Causes of weathering
Causes of weathering