MEETING SUMMARY ILCA 2020 AND ESMO 2020 VIRTUAL














- Slides: 28


MEETING SUMMARY ILCA 2020 AND ESMO 2020, VIRTUAL MEETINGS Dr. Su Pin Choo Curie Oncology and National Cancer Centre, Singapore HIGHLIGHTS FROM HCC CONNECT SEPTEMBER 2020 2

DISCLAIMER Please note: The views expressed within this presentation are the personal opinions of the author. They do not necessarily represent the views of the author’s academic institution or the rest of HCC CONNECT group. This content is supported by an Independent Educational Grant from Bayer. Dr. Su Pin Choo has the following relevant financial disclosures: • Bayer, BMS, Eisai, MSD, Roche, Sirtex HCC, hepatocellular carcinoma 3

ABSTRACTS COVERED IN THIS SUMMARY Abstract title First author Abstract number Sequential treatment with sorafenib followed by regorafenib in patients with unresectable HCC: interim analysis of the observational REFINE study Merle P ILCA 2020. Abstract P-115. Poster presentation Real-world dosing of regorafenib in patients with unresectable HCC: Interim analysis of the observational REFINE study Merle P ESMO 2020. Abstract #1010 P. Poster presentation Regorafenib in patients with unresectable HCC in real-world practice in Asia: Interim results from the observational REFINE study Lim HY ESMO 2020. Abstract #1009 P. Poster presentation Nivolumab after selective internal radiation therapy using selective internal radiation-spheres resin microspheres in patients with HCC: the NASIR-HCC trial Sangro B ILCA 2020. Abstract #O-27. Oral presentation IMbrave 150: Management of adverse events of special interest for Ikeda M atezolizumab and bevacizumab in unresectable HCC ESMO 2020. Abstract #1008 P. Poster presentation 4

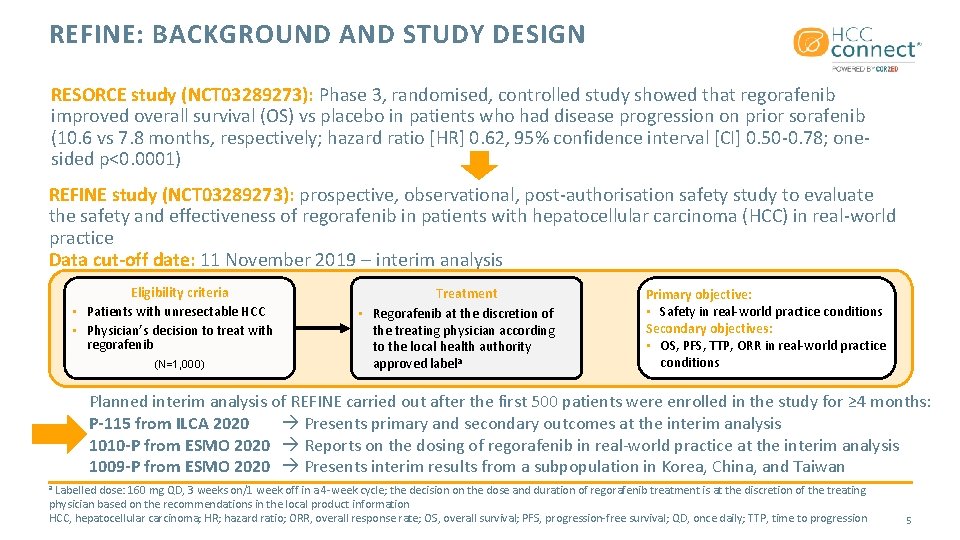
REFINE: BACKGROUND AND STUDY DESIGN RESORCE study (NCT 03289273): Phase 3, randomised, controlled study showed that regorafenib improved overall survival (OS) vs placebo in patients who had disease progression on prior sorafenib (10. 6 vs 7. 8 months, respectively; hazard ratio [HR] 0. 62, 95% confidence interval [CI] 0. 50 -0. 78; onesided p<0. 0001) REFINE study (NCT 03289273): prospective, observational, post-authorisation safety study to evaluate the safety and effectiveness of regorafenib in patients with hepatocellular carcinoma (HCC) in real-world practice Data cut-off date: 11 November 2019 – interim analysis Eligibility criteria • Patients with unresectable HCC • Physician’s decision to treat with regorafenib (N=1, 000) Treatment • Regorafenib at the discretion of the treating physician according to the local health authority approved labela Primary objective: • Safety in real-world practice conditions Secondary objectives: • OS, PFS, TTP, ORR in real-world practice conditions Planned interim analysis of REFINE carried out after the first 500 patients were enrolled in the study for ≥ 4 months: P-115 from ILCA 2020 Presents primary and secondary outcomes at the interim analysis 1010 -P from ESMO 2020 Reports on the dosing of regorafenib in real-world practice at the interim analysis 1009 -P from ESMO 2020 Presents interim results from a subpopulation in Korea, China, and Taiwan Labelled dose: 160 mg QD, 3 weeks on/1 week off in a 4 -week cycle; the decision on the dose and duration of regorafenib treatment is at the discretion of the treating physician based on the recommendations in the local product information HCC, hepatocellular carcinoma; HR; hazard ratio; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; QD, once daily; TTP, time to progression a 5

SEQUENTIAL TREATMENT WITH SORAFENIB FOLLOWED BY REGORAFENIB IN PATIENTS WITH UNRESECTABLE HCC: INTERIM ANALYSIS OF THE OBSERVATIONAL REFINE STUDY Merle P, et al. ILCA 2020. Abstract P-115. Poster presentation • HCC, hepatocellular carcinoma 6

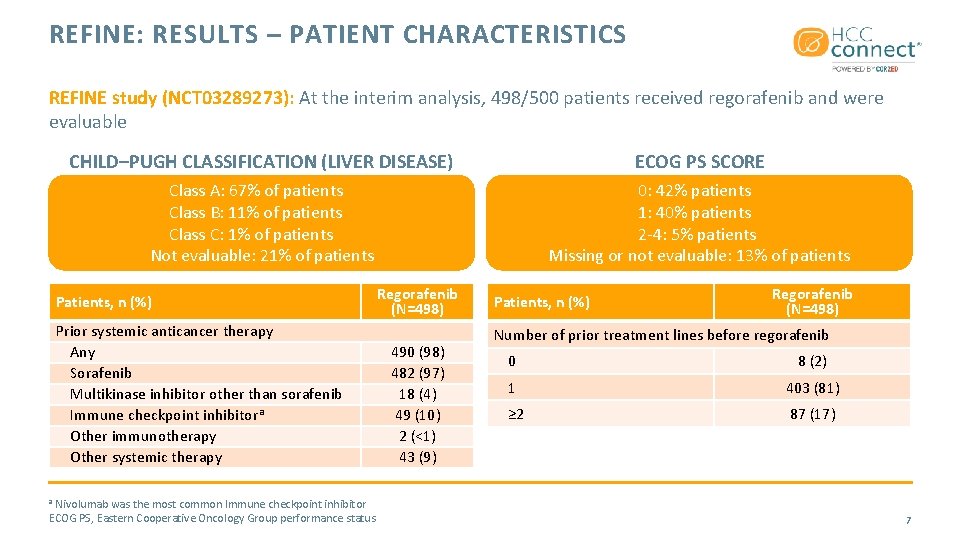
REFINE: RESULTS – PATIENT CHARACTERISTICS REFINE study (NCT 03289273): At the interim analysis, 498/500 patients received regorafenib and were evaluable CHILD–PUGH CLASSIFICATION (LIVER DISEASE) ECOG PS SCORE Class A: 67% of patients Class B: 11% of patients Class C: 1% of patients Not evaluable: 21% of patients 0: 42% patients 1: 40% patients 2 -4: 5% patients Missing or not evaluable: 13% of patients Patients, n (%) Prior systemic anticancer therapy Any Sorafenib Multikinase inhibitor other than sorafenib Immune checkpoint inhibitora Other immunotherapy Other systemic therapy Nivolumab was the most common Immune checkpoint inhibitor ECOG PS, Eastern Cooperative Oncology Group performance status Regorafenib (N=498) 490 (98) 482 (97) 18 (4) 49 (10) 2 (<1) 43 (9) Patients, n (%) Regorafenib (N=498) Number of prior treatment lines before regorafenib 0 8 (2) 1 403 (81) ≥ 2 87 (17) a 7

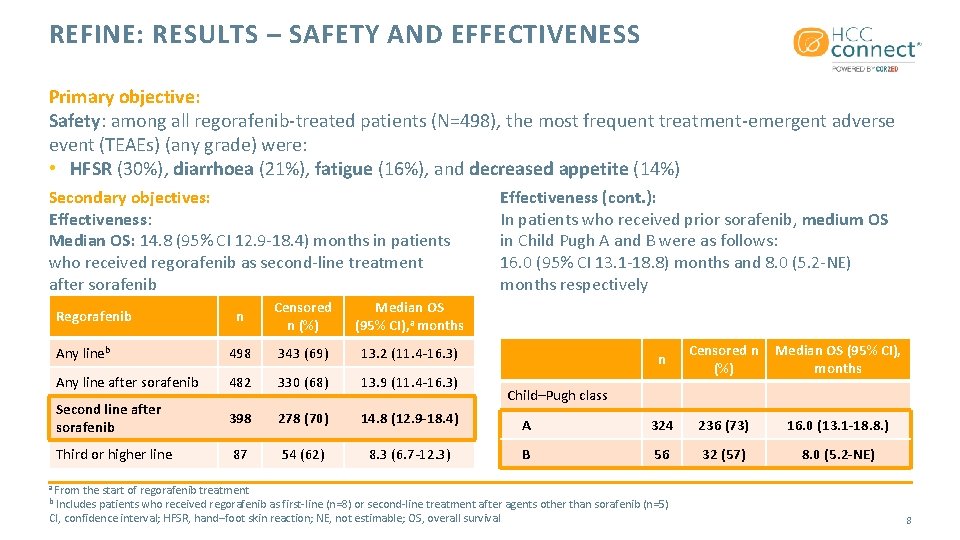
REFINE: RESULTS – SAFETY AND EFFECTIVENESS Primary objective: Safety: among all regorafenib-treated patients (N=498), the most frequent treatment-emergent adverse event (TEAEs) (any grade) were: • HFSR (30%), diarrhoea (21%), fatigue (16%), and decreased appetite (14%) Secondary objectives: Effectiveness: Median OS: 14. 8 (95% CI 12. 9 -18. 4) months in patients who received regorafenib as second-line treatment after sorafenib n Censored n (%) Median OS (95% CI), a months Any lineb 498 343 (69) 13. 2 (11. 4 -16. 3) Any line after sorafenib 482 330 (68) 13. 9 (11. 4 -16. 3) Second line after sorafenib 398 278 (70) 14. 8 (12. 9 -18. 4) Third or higher line 87 54 (62) 8. 3 (6. 7 -12. 3) Regorafenib Effectiveness (cont. ): In patients who received prior sorafenib, medium OS in Child Pugh A and B were as follows: 16. 0 (95% CI 13. 1 -18. 8) months and 8. 0 (5. 2 -NE) months respectively n Censored n (%) Median OS (95% CI), months A 324 236 (73) 16. 0 (13. 1 -18. 8. ) B 56 32 (57) 8. 0 (5. 2 -NE) Child–Pugh class a From the start of regorafenib treatment Includes patients who received regorafenib as first-line (n=8) or second-line treatment after agents other than sorafenib (n=5) CI, confidence interval; HFSR, hand–foot skin reaction; NE, not estimable; OS, overall survival b 8

REAL-WORLD DOSING OF REGORAFENIB IN PATIENTS WITH UNRESECTABLE HCC: INTERIM ANALYSIS OF THE OBSERVATIONAL REFINE STUDY Merle P, et al. ESMO 2020. Abstract #1010 P. Poster presentation • HCC, hepatocellular carcinoma 9

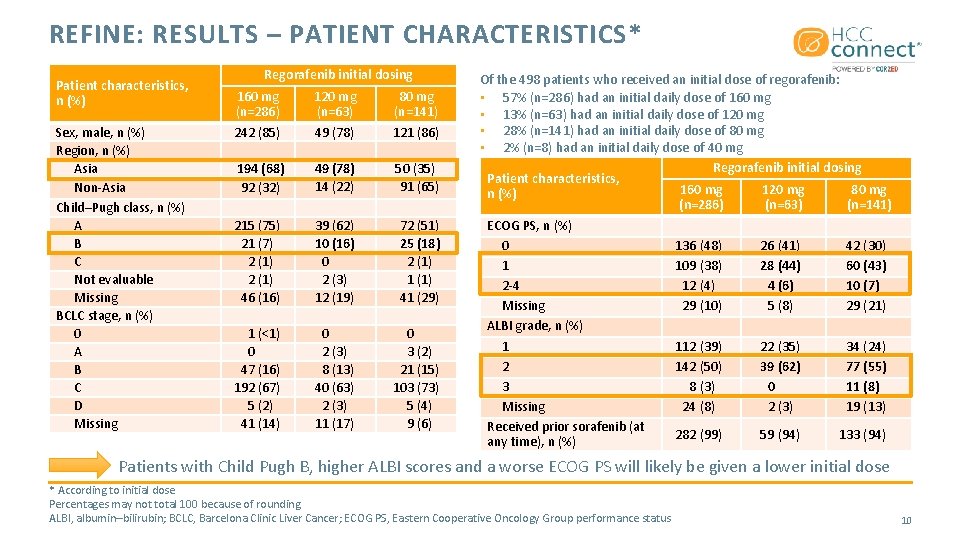
REFINE: RESULTS – PATIENT CHARACTERISTICS* Patient characteristics, n (%) Sex, male, n (%) Region, n (%) Asia Non-Asia Child–Pugh class, n (%) A B C Not evaluable Missing BCLC stage, n (%) 0 A B C D Missing Regorafenib initial dosing 160 mg (n=286) 242 (85) 120 mg (n=63) 49 (78) 80 mg (n=141) 121 (86) 194 (68) 92 (32) 49 (78) 14 (22) 50 (35) 91 (65) 215 (75) 21 (7) 2 (1) 46 (16) 39 (62) 10 (16) 0 2 (3) 12 (19) 72 (51) 25 (18) 2 (1) 1 (1) 41 (29) 1 (<1) 0 47 (16) 192 (67) 5 (2) 41 (14) 0 2 (3) 8 (13) 40 (63) 2 (3) 11 (17) 0 3 (2) 21 (15) 103 (73) 5 (4) 9 (6) Of the 498 patients who received an initial dose of regorafenib: • 57% (n=286) had an initial daily dose of 160 mg • 13% (n=63) had an initial daily dose of 120 mg • 28% (n=141) had an initial daily dose of 80 mg • 2% (n=8) had an initial daily dose of 40 mg Regorafenib initial dosing Patient characteristics, 160 mg 120 mg 80 mg n (%) (n=286) (n=63) (n=141) ECOG PS, n (%) 0 136 (48) 26 (41) 42 (30) 1 109 (38) 28 (44) 60 (43) 2 -4 12 (4) 4 (6) 10 (7) Missing 29 (10) 5 (8) 29 (21) ALBI grade, n (%) 1 112 (39) 22 (35) 34 (24) 2 142 (50) 39 (62) 77 (55) 3 8 (3) 0 11 (8) Missing 24 (8) 2 (3) 19 (13) Received prior sorafenib (at 282 (99) 59 (94) 133 (94) any time), n (%) Patients with Child Pugh B, higher ALBI scores and a worse ECOG PS will likely be given a lower initial dose * According to initial dose Percentages may not total 100 because of rounding ALBI, albumin–bilirubin; BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status 10

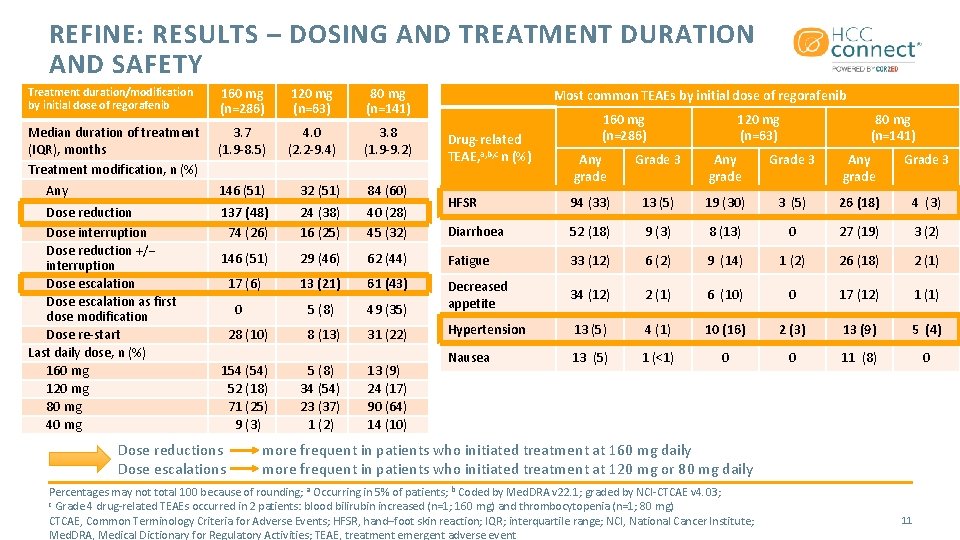
REFINE: RESULTS – DOSING AND TREATMENT DURATION AND SAFETY Treatment duration/modification by initial dose of regorafenib 160 mg (n=286) 120 mg (n=63) 80 mg (n=141) Median duration of treatment (IQR), months Treatment modification, n (%) Any 3. 7 (1. 9 -8. 5) 4. 0 (2. 2 -9. 4) 3. 8 (1. 9 -9. 2) 146 (51) 32 (51) 84 (60) Dose reduction Dose interruption Dose reduction +/− interruption Dose escalation as first dose modification Dose re-start Last daily dose, n (%) 160 mg 120 mg 80 mg 40 mg 137 (48) 74 (26) 24 (38) 16 (25) 40 (28) 45 (32) 146 (51) 29 (46) 17 (6) Most common TEAEs by initial dose of regorafenib Drug-related TEAE, a, b, c n (%) 160 mg (n=286) 120 mg (n=63) 80 mg (n=141) Any grade Grade 3 HFSR 94 (33) 13 (5) 19 (30) 3 (5) 26 (18) 4 (3) Diarrhoea 52 (18) 9 (3) 8 (13) 0 27 (19) 3 (2) 62 (44) Fatigue 33 (12) 6 (2) 9 (14) 1 (2) 26 (18) 2 (1) 13 (21) 61 (43) 5 (8) 49 (35) Decreased appetite 34 (12) 2 (1) 6 (10) 0 17 (12) 1 (1) 28 (10) 8 (13) 31 (22) Hypertension 13 (5) 4 (1) 10 (16) 2 (3) 13 (9) 5 (4) 154 (54) 52 (18) 71 (25) 9 (3) 5 (8) 34 (54) 23 (37) 1 (2) 13 (9) 24 (17) 90 (64) 14 (10) Nausea 13 (5) 1 (<1) 0 0 11 (8) 0 0 Dose reductions Dose escalations more frequent in patients who initiated treatment at 160 mg daily more frequent in patients who initiated treatment at 120 mg or 80 mg daily Percentages may not total 100 because of rounding; a Occurring in 5% of patients; b Coded by Med. DRA v 22. 1; graded by NCI-CTCAE v 4. 03; c Grade 4 drug-related TEAEs occurred in 2 patients: blood bilirubin increased (n=1; 160 mg) and thrombocytopenia (n=1; 80 mg) CTCAE, Common Terminology Criteria for Adverse Events; HFSR, hand–foot skin reaction; IQR; interquartile range; NCI, National Cancer Institute; Med. DRA, Medical Dictionary for Regulatory Activities; TEAE, treatment emergent adverse event 11

REGORAFENIB IN PATIENTS WITH UNRESECTABLE HCC IN REAL-WORLD PRACTICE IN ASIA: INTERIM RESULTS FROM THE OBSERVATIONAL REFINE STUDY Lim HY, et al. ESMO 2020. Abstract #1009 P. Poster presentation • HCC, hepatocellular carcinoma 12

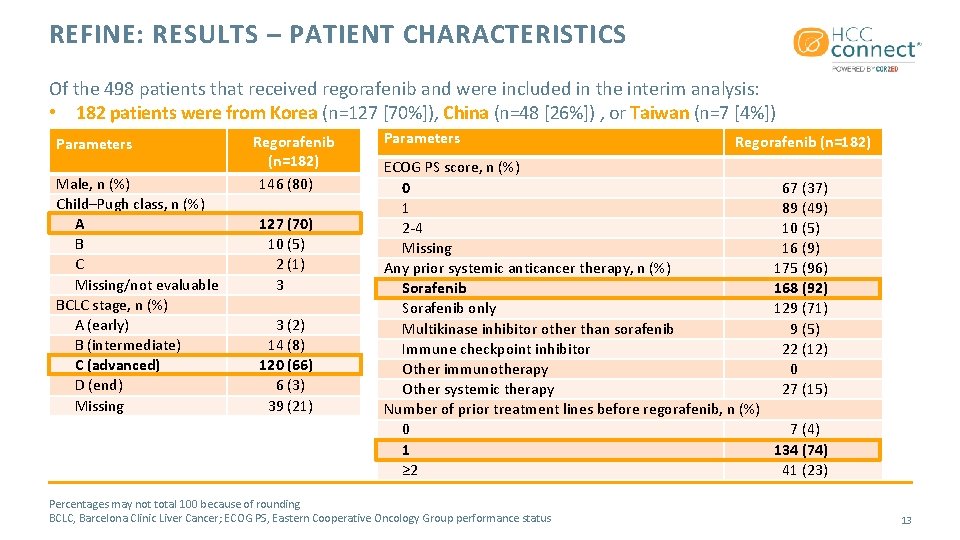
REFINE: RESULTS – PATIENT CHARACTERISTICS Of the 498 patients that received regorafenib and were included in the interim analysis: • 182 patients were from Korea (n=127 [70%]), China (n=48 [26%]) , or Taiwan (n=7 [4%]) Parameters Male, n (%) Child–Pugh class, n (%) A B C Missing/not evaluable BCLC stage, n (%) A (early) B (intermediate) C (advanced) D (end) Missing Regorafenib (n=182) 146 (80) 127 (70) 10 (5) 2 (1) 3 3 (2) 14 (8) 120 (66) 6 (3) 39 (21) Parameters Regorafenib (n=182) ECOG PS score, n (%) 0 1 2 -4 Missing Any prior systemic anticancer therapy, n (%) Sorafenib only Multikinase inhibitor other than sorafenib Immune checkpoint inhibitor Other immunotherapy Other systemic therapy Number of prior treatment lines before regorafenib, n (%) 0 1 ≥ 2 Percentages may not total 100 because of rounding BCLC, Barcelona Clinic Liver Cancer; ECOG PS, Eastern Cooperative Oncology Group performance status 67 (37) 89 (49) 10 (5) 16 (9) 175 (96) 168 (92) 129 (71) 9 (5) 22 (12) 0 27 (15) 7 (4) 134 (74) 41 (23) 13

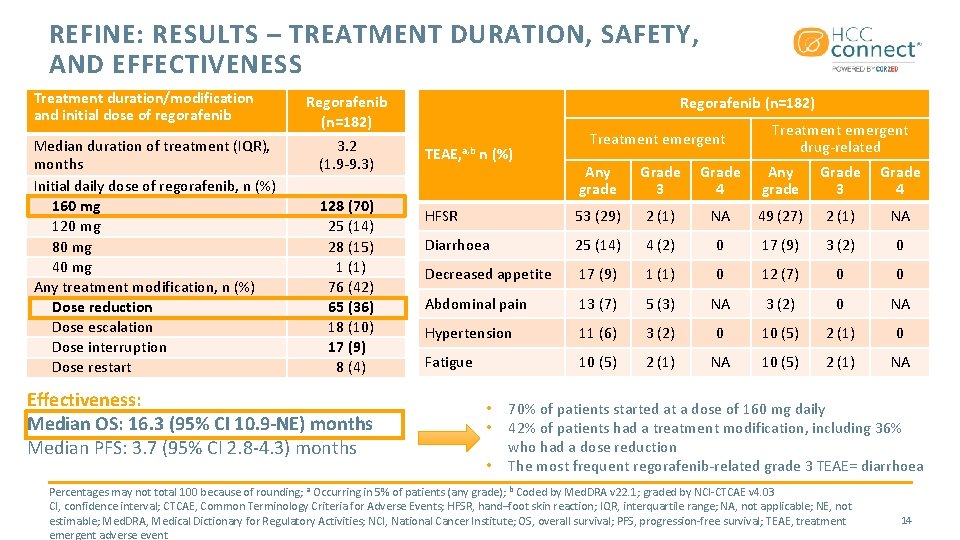
REFINE: RESULTS – TREATMENT DURATION, SAFETY, AND EFFECTIVENESS Treatment duration/modification and initial dose of regorafenib Median duration of treatment (IQR), months Initial daily dose of regorafenib, n (%) 160 mg 120 mg 80 mg 40 mg Any treatment modification, n (%) Dose reduction Dose escalation Dose interruption Dose restart Regorafenib (n=182) 3. 2 (1. 9 -9. 3) 128 (70) 25 (14) 28 (15) 1 (1) 76 (42) 65 (36) 18 (10) 17 (9) 8 (4) Effectiveness: Median OS: 16. 3 (95% CI 10. 9 -NE) months Median PFS: 3. 7 (95% CI 2. 8 -4. 3) months Regorafenib (n=182) TEAE, a, b n (%) Treatment emergent drug-related Any grade Grade 3 Grade 4 HFSR 53 (29) 2 (1) NA 49 (27) 2 (1) NA Diarrhoea 25 (14) 4 (2) 0 17 (9) 3 (2) 0 Decreased appetite 17 (9) 1 (1) 0 12 (7) 0 0 Abdominal pain 13 (7) 5 (3) NA 3 (2) 0 NA Hypertension 11 (6) 3 (2) 0 10 (5) 2 (1) 0 Fatigue 10 (5) 2 (1) NA • • • 70% of patients started at a dose of 160 mg daily 42% of patients had a treatment modification, including 36% who had a dose reduction The most frequent regorafenib-related grade 3 TEAE= diarrhoea Percentages may not total 100 because of rounding; a Occurring in 5% of patients (any grade); b Coded by Med. DRA v 22. 1; graded by NCI-CTCAE v 4. 03 CI, confidence interval; CTCAE, Common Terminology Criteria for Adverse Events; HFSR, hand–foot skin reaction; IQR, interquartile range; NA, not applicable; NE, not estimable; Med. DRA, Medical Dictionary for Regulatory Activities; NCI, National Cancer Institute; OS, overall survival; PFS, progression-free survival; TEAE, treatment emergent adverse event 14

REFINE: CONCLUSIONS KEY FINDINGS Patient population is broader in the REFINE study compared to the RESORCE trial: • Inclusion of Child–Pugh class B liver disease • Patients with ECOG PS score ≥ 2 Primary objective: • Safety profile of regorafenib consistent with RESORCE study with no unexpected safety signals Secondary objectives: • Effectiveness of regorafenib (median OS) consistent with RESORCE study results Dosing of regorafenib: • Most patients (57%) initiated regorafenib at the label dose of 160 mg QD • Median duration of treatment and safety profile was similar in patients who initiated treatment at 160 mg, 120 mg, and 80 mg QD Safety and effectiveness in Korean, Taiwanese, and Chinese population: • Longer median OS in this population (16. 3 months) vs RESORCE (regorafenib arm: 10. 6 months) vs REFINE global cohort (13. 2 months) but the proportion of censored patients was high • Safety profile in this sub-population was consistent with the one in the REFINE global cohort ECOG PS, Eastern Cooperative Oncology Group performance status; OS, overall survival; QD, once daily 15

REFINE: DISCUSSION Impact of real-world data on clinical practice and perspectives In real-world practice, regorafenib showed a consistent safety profile and effectiveness in broader population not usually included in clinical trial population: – Patients with a poor ECOG PS score (≥ 2) – Patients with Child–Pugh class B liver function ECOG PS, Eastern Cooperative Oncology Group performance status; HCC, hepatocellular carcinoma; QD, once daily 16

NIVOLUMAB AFTER SELECTIVE INTERNAL RADIATION THERAPY USING SELECTIVE INTERNAL RADIATION-SPHERES RESIN MICROSPHERES IN PATIENTS WITH HCC: THE NASIR-HCC TRIAL Sangro B, et al. ILCA 2020. Abstract #O-27. Oral presentation • HCC, hepatocellular carcinoma 17

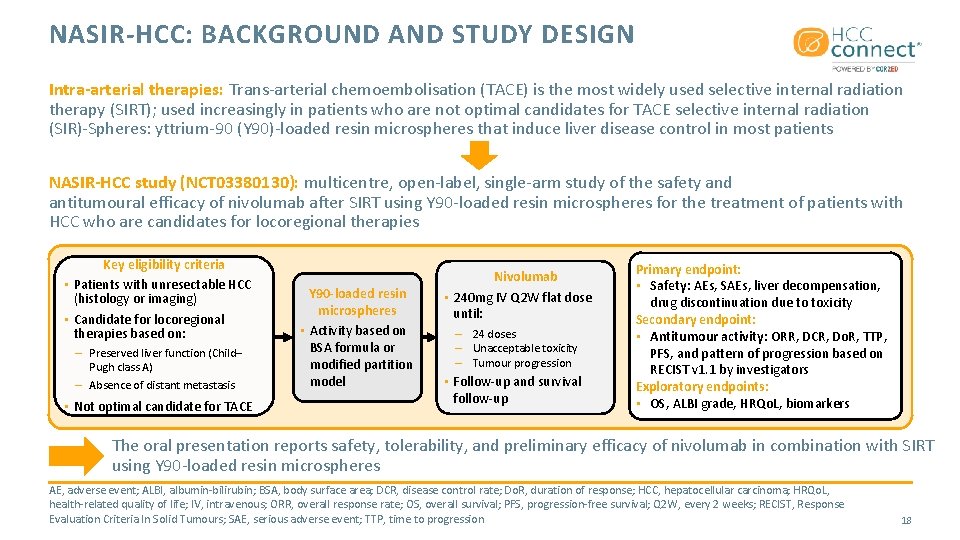
NASIR-HCC: BACKGROUND AND STUDY DESIGN Intra-arterial therapies: Trans-arterial chemoembolisation (TACE) is the most widely used selective internal radiation therapy (SIRT); used increasingly in patients who are not optimal candidates for TACE selective internal radiation (SIR)-Spheres: yttrium-90 (Y 90)-loaded resin microspheres that induce liver disease control in most patients NASIR-HCC study (NCT 03380130): multicentre, open-label, single-arm study of the safety and antitumoural efficacy of nivolumab after SIRT using Y 90 -loaded resin microspheres for the treatment of patients with HCC who are candidates for locoregional therapies Key eligibility criteria • Patients with unresectable HCC (histology or imaging) • Candidate for locoregional therapies based on: – Preserved liver function (Child– Pugh class A) – Absence of distant metastasis • Not optimal candidate for TACE Y 90 -loaded resin microspheres • Activity based on BSA formula or modified partition model Nivolumab • 240 mg IV Q 2 W flat dose until: – 24 doses – Unacceptable toxicity – Tumour progression • Follow-up and survival follow-up Primary endpoint: • Safety: AEs, SAEs, liver decompensation, drug discontinuation due to toxicity Secondary endpoint: • Antitumour activity: ORR, DCR, Do. R, TTP, PFS, and pattern of progression based on RECIST v 1. 1 by investigators Exploratory endpoints: • OS, ALBI grade, HRQo. L, biomarkers The oral presentation reports safety, tolerability, and preliminary efficacy of nivolumab in combination with SIRT using Y 90 -loaded resin microspheres AE, adverse event; ALBI, albumin-bilirubin; BSA, body surface area; DCR, disease control rate; Do. R, duration of response; HCC, hepatocellular carcinoma; HRQo. L, health-related quality of life; IV, intravenous; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; Q 2 W, every 2 weeks; RECIST, Response Evaluation Criteria In Solid Tumours; SAE, serious adverse event; TTP, time to progression 18

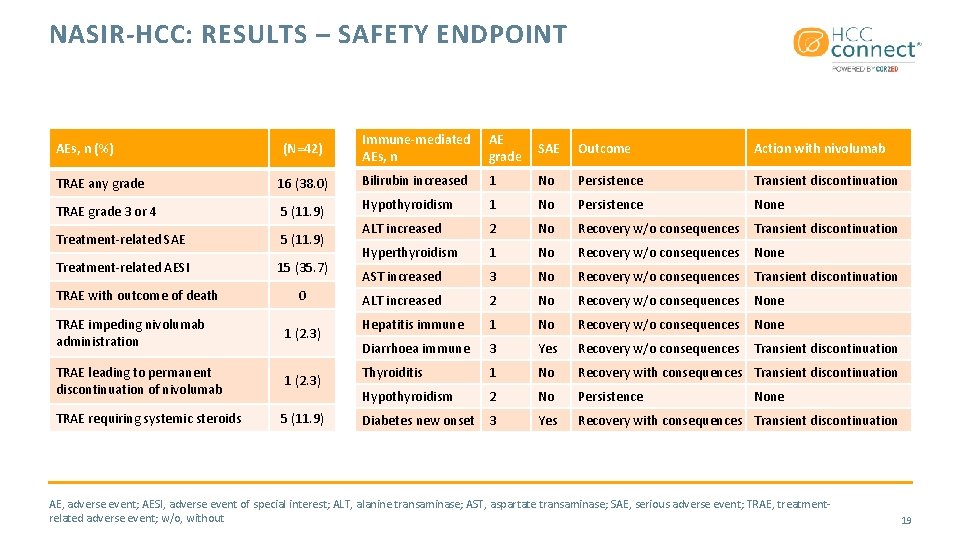
NASIR-HCC: RESULTS – SAFETY ENDPOINT (N=42) Immune-mediated AEs, n AE grade SAE Outcome Action with nivolumab TRAE any grade 16 (38. 0) Bilirubin increased 1 No Persistence Transient discontinuation TRAE grade 3 or 4 5 (11. 9) Hypothyroidism 1 No Persistence None Treatment-related SAE 5 (11. 9) ALT increased 2 No Recovery w/o consequences Transient discontinuation Treatment-related AESI 15 (35. 7) Hyperthyroidism 1 No Recovery w/o consequences None AST increased 3 No Recovery w/o consequences Transient discontinuation ALT increased 2 No Recovery w/o consequences None Hepatitis immune 1 No Recovery w/o consequences None Diarrhoea immune 3 Yes Recovery w/o consequences Transient discontinuation Thyroiditis 1 No Recovery with consequences Transient discontinuation Hypothyroidism 2 No Persistence Diabetes new onset 3 Yes Recovery with consequences Transient discontinuation AEs, n (%) TRAE with outcome of death 0 TRAE impeding nivolumab administration 1 (2. 3) TRAE leading to permanent discontinuation of nivolumab 1 (2. 3) TRAE requiring systemic steroids 5 (11. 9) None AE, adverse event; AESI, adverse event of special interest; ALT, alanine transaminase; AST, aspartate transaminase; SAE, serious adverse event; TRAE, treatmentrelated adverse event; w/o, without 19

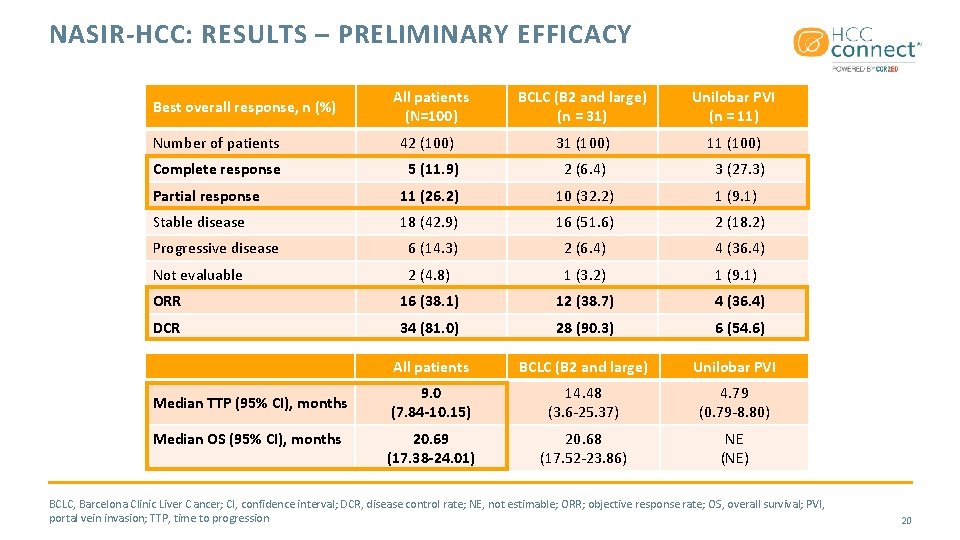
NASIR-HCC: RESULTS – PRELIMINARY EFFICACY All patients (N=100) BCLC (B 2 and large) (n = 31) Unilobar PVI (n = 11) Number of patients 42 (100) 31 (100) 11 (100) Complete response 5 (11. 9) 2 (6. 4) 3 (27. 3) Partial response 11 (26. 2) 10 (32. 2) 1 (9. 1) Stable disease 18 (42. 9) 16 (51. 6) 2 (18. 2) Progressive disease 6 (14. 3) 2 (6. 4) 4 (36. 4) Not evaluable 2 (4. 8) 1 (3. 2) 1 (9. 1) ORR 16 (38. 1) 12 (38. 7) 4 (36. 4) DCR 34 (81. 0) 28 (90. 3) 6 (54. 6) All patients BCLC (B 2 and large) Unilobar PVI 9. 0 (7. 84 -10. 15) 14. 48 (3. 6 -25. 37) 4. 79 (0. 79 -8. 80) 20. 69 (17. 38 -24. 01) 20. 68 (17. 52 -23. 86) NE (NE) Best overall response, n (%) Median TTP (95% CI), months Median OS (95% CI), months BCLC, Barcelona Clinic Liver C ancer; CI, confidence interval; DCR, disease control rate; NE, not estimable; ORR; objective response rate; OS, overall survival; PVI, portal vein invasion; TTP, time to progression 20

NASIR-HCC: CONCLUSIONS KEY FINDINGS • Safety profile of nivolumab in combination with SIRT using Y 90 -loaded resin microspheres is favourable • The preliminary efficacy data look promising and warrant further investigation in a controlled trial setting PERSPECTIVES • These data are promising and may lead to novel treatment approaches for patients with intermediate stage HCC, hepatocellular carcinoma; PVI, portal vein invasion; TTP, time to progression; SIRT, selective internal radiation therapy; Y 90, yttrium-90 21

IMBRAVE 150: MANAGEMENT OF ADVERSE EVENTS OF SPECIAL INTEREST FOR ATEZOLIZUMAB AND BEVACIZUMAB IN UNRESECTABLE HCC Ikeda M, et al. ESMO 2020. Abstract #1008 P. Poster presentation • HCC, hepatocellular carcinoma 22

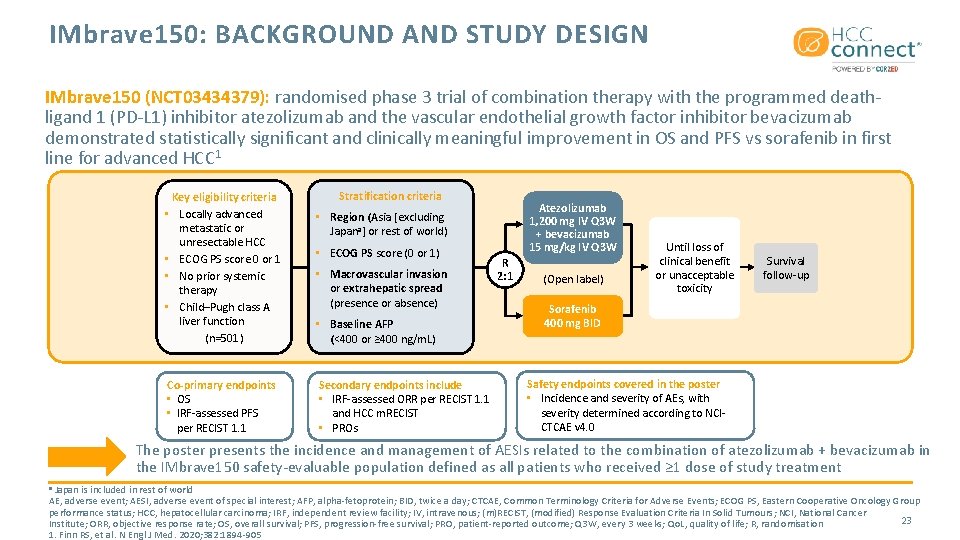
IMbrave 150: BACKGROUND AND STUDY DESIGN IMbrave 150 (NCT 03434379): randomised phase 3 trial of combination therapy with the programmed deathligand 1 (PD-L 1) inhibitor atezolizumab and the vascular endothelial growth factor inhibitor bevacizumab demonstrated statistically significant and clinically meaningful improvement in OS and PFS vs sorafenib in first line for advanced HCC 1 Key eligibility criteria • Locally advanced metastatic or unresectable HCC • ECOG PS score 0 or 1 • No prior systemic therapy • Child–Pugh class A liver function (n=501) Co-primary endpoints • OS • IRF-assessed PFS per RECIST 1. 1 Stratification criteria Atezolizumab 1, 200 mg IV Q 3 W + bevacizumab 15 mg/kg IV Q 3 W • Region (Asia [excluding Japana] or rest of world) • ECOG PS score (0 or 1) • Macrovascular invasion or extrahepatic spread (presence or absence) • Baseline AFP (<400 or ≥ 400 ng/m. L) Secondary endpoints include • IRF-assessed ORR per RECIST 1. 1 and HCC m. RECIST • PROs R 2: 1 (Open label) Until loss of clinical benefit or unacceptable toxicity Survival follow-up Sorafenib 400 mg BID Safety endpoints covered in the poster • Incidence and severity of AEs, with severity determined according to NCICTCAE v 4. 0 The poster presents the incidence and management of AESIs related to the combination of atezolizumab + bevacizumab in the IMbrave 150 safety-evaluable population defined as all patients who received ≥ 1 dose of study treatment Japan is included in rest of world AE, adverse event; AESI, adverse event of special interest; AFP, alpha-fetoprotein; BID, twice a day; CTCAE, Common Terminology Criteria for Adverse Events; ECOG PS, Eastern Cooperative Oncology Group performance status; HCC, hepatocellular carcinoma; IRF, independent review facility; IV, intravenous; (m)RECIST, (modified) Response Evaluation Criteria In Solid Tumours; NCI, National Cancer 23 Institute; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PRO, patient-reported outcome; Q 3 W, every 3 weeks; Qo. L, quality of life; R, randomisation 1. Finn RS, et al. N Engl J Med. 2020; 382: 1894 -905 a

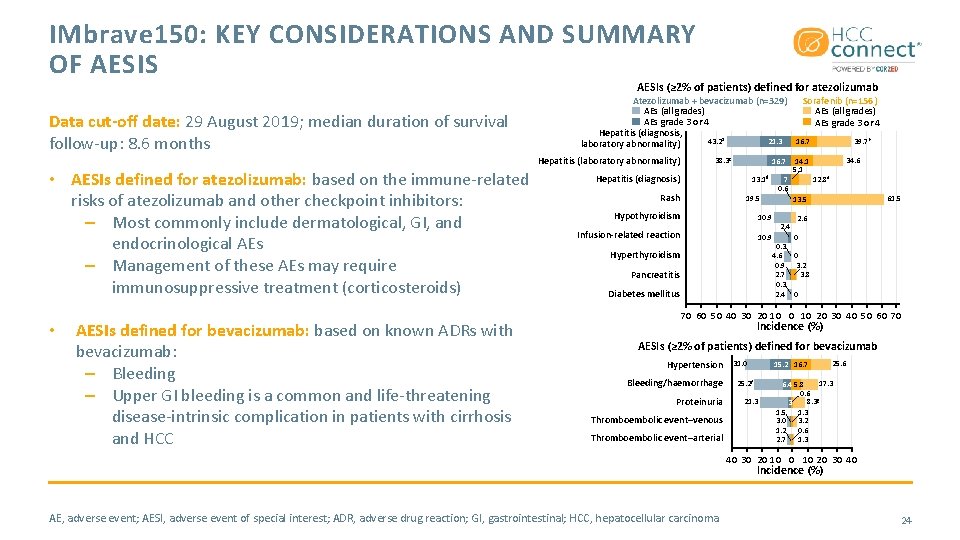
IMbrave 150: KEY CONSIDERATIONS AND SUMMARY OF AESIS AESIs (≥ 2% of patients) defined for atezolizumab Data cut-off date: 29 August 2019; median duration of survival follow-up: 8. 6 months Atezolizumab + bevacizumab (n=329) AEs (all grades) AEs grade 3 or 4 Hepatitis (diagnosis, 43. 2 a 21. 3 laboratory abnormality) Hepatitis (laboratory abnormality) • AESIs defined for atezolizumab: based on the immune-related risks of atezolizumab and other checkpoint inhibitors: – Most commonly include dermatological, GI, and endocrinological AEs – Management of these AEs may require immunosuppressive treatment (corticosteroids) • AESIs defined for bevacizumab: based on known ADRs with bevacizumab: – Bleeding – Upper GI bleeding is a common and life-threatening disease-intrinsic complication in patients with cirrhosis and HCC 38. 3 c 16. 7 Hepatitis (diagnosis) 13. 1 d Rash 10. 9 Infusion-related reaction 10. 9 Hyperthyroidism Pancreatitis Diabetes mellitus 39. 7 b 16. 7 14. 1 5. 1 7 0. 6 19. 5 Hypothyroidism Sorafenib (n=156) AEs (all grades) AEs grade 3 or 4 34. 6 12. 8 e 61. 5 13. 5 2. 4 0. 3 4. 6 0. 9 2. 7 0. 3 2. 4 2. 6 0 0 3. 2 3. 8 0 70 60 50 40 30 20 10 20 30 40 50 60 70 Incidence (%) AESIs (≥ 2% of patients) defined for bevacizumab Hypertension Bleeding/haemorrhage Proteinuria Thromboembolic event–venous Thromboembolic event–arterial 31. 0 15. 2 16. 7 25. 2 f 21. 3 25. 6 17. 3 6. 4 5. 8 0. 6 8. 3 g 3 1. 5 1. 3 3. 0 3. 2 1. 2 0. 6 2. 7 1. 3 40 30 20 10 20 30 40 Incidence (%) AE, adverse event; AESI, adverse event of special interest; ADR, adverse drug reaction; GI, gastrointestinal; HCC, hepatocellular carcinoma 24

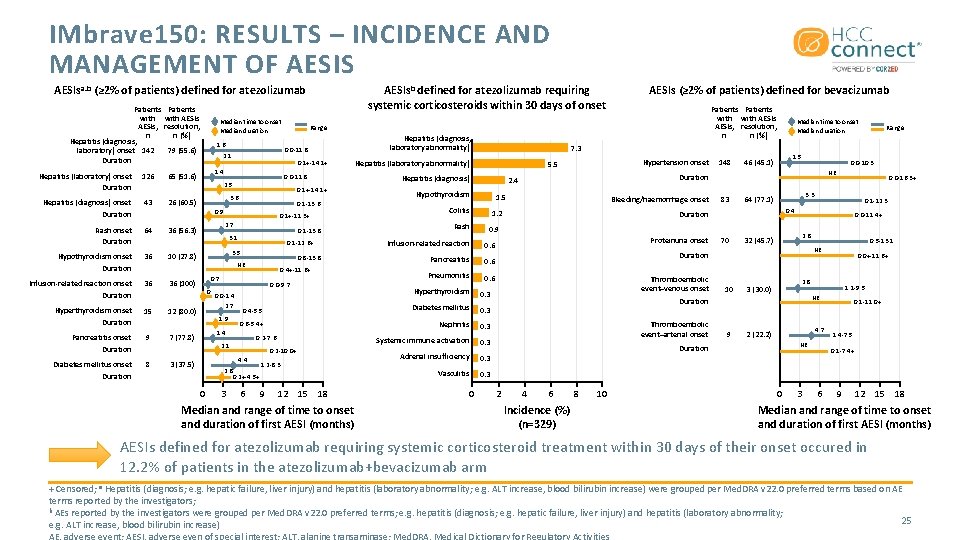
IMbrave 150: RESULTS – INCIDENCE AND MANAGEMENT OF AESIS AESIsb defined for atezolizumab requiring systemic corticosteroids within 30 days of onset AESIsa. b (≥ 2% of patients) defined for atezolizumab Patients with AESIs, n Hepatitis (diagnosis, laboratory) onset 142 Duration Hepatitis (laboratory) onset Duration 126 Hepatitis (diagnosis) onset Duration 43 Rash onset Duration 64 Patients with AESIs resolution, n (%) Median time to onset Median duration 1. 6 79 (55. 6) 10 (27. 8) Infusion-related reaction onset 36 36 (100) 15 Pancreatitis onset Duration 9 Diabetes mellitus onset Duration 8 0. 1 -13. 6 0. 9 0. 1+-11. 3+ 2. 7 0. 1 -13. 8 3. 1 0. 1 -12. 6+ 3. 5 0. 8 -13. 8 NE 0. 4+-11. 6+ 0. 7 0 Duration Hyperthyroidism onset Duration 0. 1+-14. 1+ 3. 6 36 (56. 3) 36 0. 0 -11. 8 2. 5 26 (60. 5) Hypothyroidism onset Duration 0. 1+-14. 1+ 1. 4 65 (51. 6) 12 (80. 0) 0. 0 -9. 7 0. 0 -1. 4 2. 7 1. 9 7 (77. 8) Hypothyroidism 1. 5 Colitis Infusion-related reaction 0. 6 Pancreatitis 0. 6 Pneumonitis 0. 6 0. 3 Systemic immune activation 0. 3 Adrenal insufficiency 0. 3 Vasculitis 0. 3 6 9 12 15 18 Median and range of time to onset and duration of first AESI (months) 0 1. 5 46 (45. 1) 83 0. 0 -10. 3 70 0. 0 -11. 4+ 2. 8 32 (45. 7) 10 9 4 6 Incidence (%) (n=329) 8 10 0. 0+-11. 6+ 2. 8 3 (30. 0) 1. 1 -9. 5 NE 4. 7 2 (22. 2) NE Duration 2 0. 5 -13. 1 NE Duration Thromboembolic event–arterial onset 0. 1 -12. 3 0. 4 Duration Thromboembolic event–venous onset 0. 0 -16. 3+ 3. 3 64 (77. 1) Duration Proteinuria onset Range NE 0. 9 Nephritis 0. 2 -10. 0+ Bleeding/haemorrhage onset 1. 2 0. 6 -5. 4+ 1. 2 -8. 3 148 Duration 2. 4 0. 3 2. 8 0. 2+-4. 5+ 3 Hepatitis (diagnosis) Diabetes mellitus 0. 2 -7. 6 Hypertension onset 5. 5 0. 4 -5. 5 4. 4 0 Hepatitis (laboratory abnormality) Rash Median time to onset Median duration 7. 3 0. 3 2. 1 3 (37. 5) Hepatitis (diagnosis, laboratory abnormality) Hyperthyroidism 1. 4 Patients with AESIs, resolution, n n (%) Range 0. 0 -11. 8 2. 1 AESIs (≥ 2% of patients) defined for bevacizumab 0 3 0. 1 -11. 0+ 1. 4 -7. 5 0. 1 -7. 4+ 6 9 12 15 18 Median and range of time to onset and duration of first AESI (months) AESIs defined for atezolizumab requiring systemic corticosteroid treatment within 30 days of their onset occured in 12. 2% of patients in the atezolizumab+bevacizumab arm + Censored; a Hepatitis (diagnosis; e. g. hepatic failure, liver injury) and hepatitis (laboratory abnormality; e. g. ALT increase, blood bilirubin increase) were grouped per Med. DRA v 22. 0 preferred terms based on AE terms reported by the investigators; b AEs reported by the investigators were grouped per Med. DRA v 22. 0 preferred terms; e. g. hepatitis (diagnosis; e. g. hepatic failure, liver injury) and hepatitis (laboratory abnormality; 25 e. g. ALT increase, blood bilirubin increase) AE, adverse event; AESI, adverse even of special interest; ALT, alanine transaminase; Med. DRA, Medical Dictionary for Regulatory Activities

IMbrave 150: CONCLUSION AND DISCUSSION KEY FINDINGS • AESIs from the IMbrave 150 study seem manageable and their severity is consistent with the known safety profiles of atezolizumab and bevacizumab individually PERSPECTIVES • The combination of atezolizumab and bevacizumab as the first-line standard of care is reinforced by the similar safety profile for the combination and individual agents AESI, adverse event of special interest 26

REACH HCC CONNECT VIA TWITTER, LINKEDIN, VIMEO, AND EMAIL OR VISIT THE GROUP’S WEBSITE http: //www. hccconnect. info Follow us on Twitter @hccconnectinfo Follow the HCC CONNECT group on Linked. In Watch us on the Vimeo Channel HCC CONNECT Email froukje. sosef@cor 2 ed. com 27

HCC CONNECT Bodenackerstrasse 17 4103 Bottmingen SWITZERLAND Dr. Froukje Sosef MD +31 6 2324 3636 froukje. sosef@cor 2 ed. com Dr. Antoine Lacombe Pharm D, MBA +41 79 529 42 79 antoine. lacombe@cor 2 ed. com Heading to the heart of Independent Medical Education Since 2012
 Esmo scale
Esmo scale Pulmn
Pulmn Esmo
Esmo Esmo declaration of interest
Esmo declaration of interest Esmo guidelines thyroid cancer
Esmo guidelines thyroid cancer What is meeting and types of meeting
What is meeting and types of meeting Types of meeting
Types of meeting For todays meeting
For todays meeting Today meeting or today's meeting
Today meeting or today's meeting Has virtual functions and accessible non-virtual destructor
Has virtual functions and accessible non-virtual destructor Joanne chien
Joanne chien Virtual meeting notice
Virtual meeting notice Nrg oncology meeting 2016
Nrg oncology meeting 2016 Virtual meeting etiquette tips
Virtual meeting etiquette tips American psychiatric association annual meeting 2020
American psychiatric association annual meeting 2020 Herbalife opportunity
Herbalife opportunity Rhic ags users meeting 2020
Rhic ags users meeting 2020 Virtual friendship and the new narcissism summary
Virtual friendship and the new narcissism summary Education and training act 2020 summary
Education and training act 2020 summary Fsa test design summary 2020
Fsa test design summary 2020 Test design summary
Test design summary Unit 5 meeting individual care and support needs assignment
Unit 5 meeting individual care and support needs assignment Child and family team meeting template
Child and family team meeting template Did mike visit his grandmother last night
Did mike visit his grandmother last night Judy and liz at last month's meeting
Judy and liz at last month's meeting Meeting agenda welcome and introductions
Meeting agenda welcome and introductions Meeting traffic
Meeting traffic Command and staff meeting
Command and staff meeting Agenda welcome and introductions
Agenda welcome and introductions