Medicines Safety Mary R Couper Medical Officer Quality

















- Slides: 23

Medicines Safety Mary R. Couper Medical Officer Quality Assurance and Safety: Medicines, WHO 2/22/2021

Medicine Safety z To undergo treatment you have to be very healthy, because apart from your sickness you have to stand the medicine. Molière 2/22/2021 WHO - EDM

Risk z. No medicinal drug is entirely or absolutely safe for all people, in all places, at all times. We must always live with some measure of uncertainty. 2/22/2021 WHO - EDM

The magnitude of the problem z. During last decades it has been demonstrated by a number of studies that medicine morbidity and mortality is one of the major health problems which is beginning to be recognized by health professionals and the public. 2/22/2021 WHO - EDM

Financial implications z. In addition suitable services to treat ADRs have a high financial burden on health care due to the hospital care of patients with drug related problems. Some countries spend up to 15 -20% of their hospital budget dealing with drug complications. 2/22/2021 WHO - EDM

Vigilance z. Vigilare = to watch yalert watchfulness yforbearance of sleep; wakefulness ywatchfulness in respect of danger; care; caution; circumspection ythe process of paying close and continuous attention 2/22/2021 WHO - EDM

Pharmacovigilance z. The science and activities relating to the detection, evaluation, understanding and prevention of adverse drug reactions or any other drug-related problems 2/22/2021 WHO - EDM

Task of Pharmacovigilance z. The task of pharmacovigilance is to define and reduce risk and harm as far as is humanly possible. 2/22/2021 WHO - EDM

Aims of Pharmacovigilance The ultimate aims of pharmacovigilance are: yto improve patient safety in relation to use yto improve public health and safety yto contribute to assessment of benefit and risk yto encourage safe, rational and more effective use yto promote understanding, education, training and communication 2/22/2021 WHO - EDM

2/22/2021 WHO - EDM

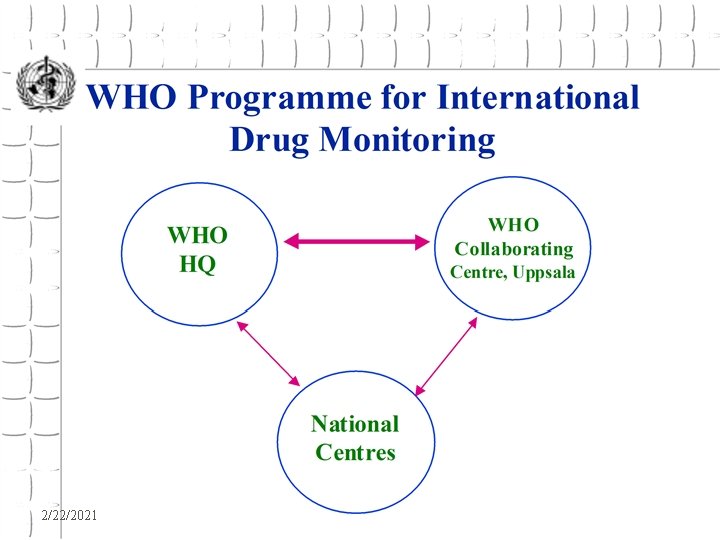
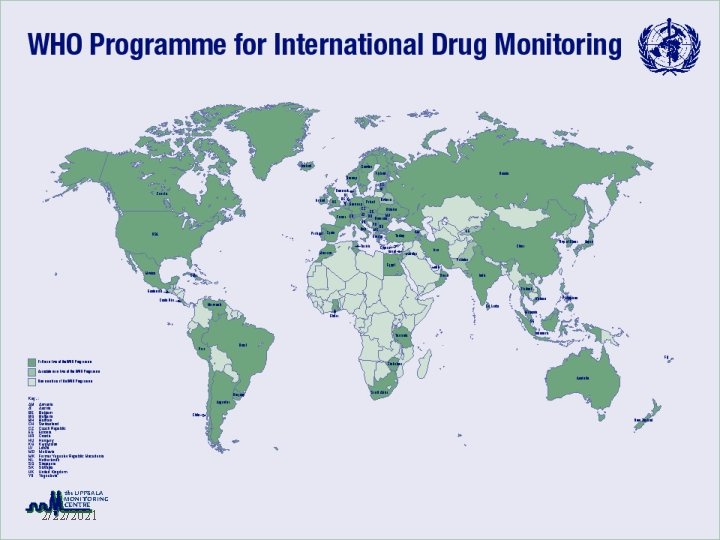
WHO Programme for International Drug Monitoring z. Exchange of Information z. Provision of technical support to countries in all aspects of pharmacovigilance z. Collection and analysis of adverse drug reactions 2/22/2021 WHO - EDM

Exchange of Information z. WHO Pharmaceuticals Newsletter z. WHO Drug Alerts z. WHO Drug Information z. WHO Restricted List z. Vigimed - electronic exchange z. Uppsala Reports 2/22/2021 WHO - EDM

Technical Support to countries z. Technical guidelines on all aspects of pharmacovigilance z. Training courses on pharmacovigilance z. Strengthening active surveillance systems z. Support to countries participating in the Programme 2/22/2021 WHO - EDM

2/22/2021 WHO - EDM

Collection and Analysis of Adverse drug Reaction Reports z. Input y. ADR Reports from National Centres z. Processing y. At the WHO Collaborating Centre for International Drug Monitoring z. Output y. Feedback to National Centres y. Signal documents 2/22/2021 WHO - EDM

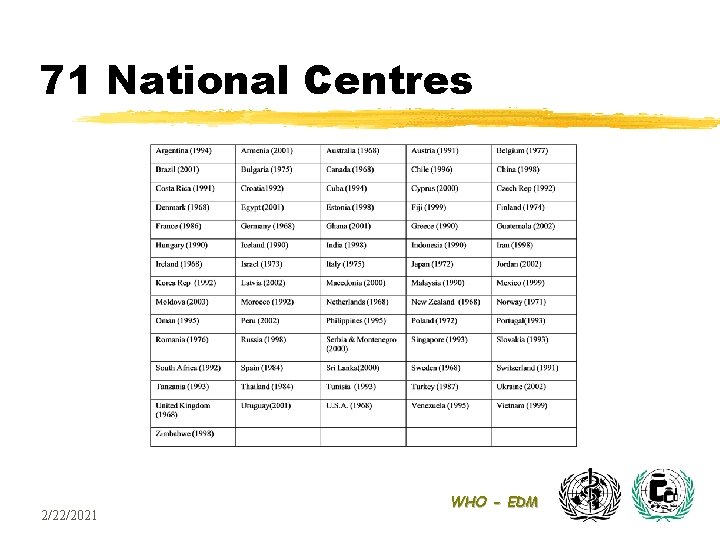
71 National Centres 2/22/2021 WHO - EDM

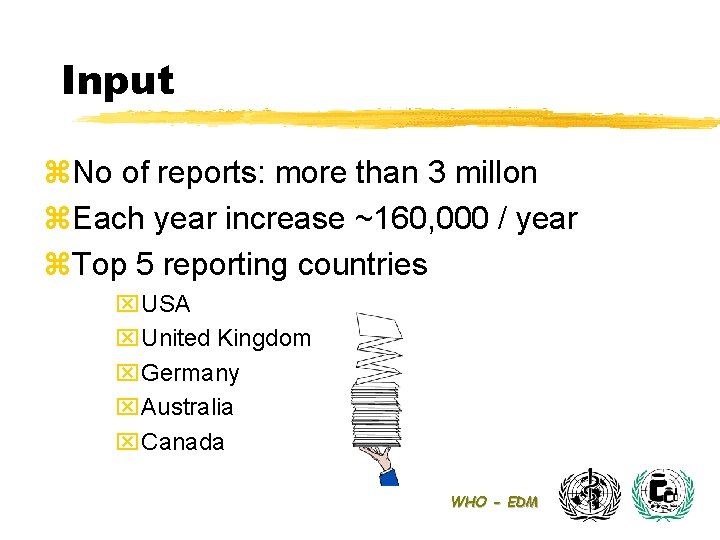
Input z. No of reports: more than 3 millon z. Each year increase ~160, 000 / year z. Top 5 reporting countries x. USA x. United Kingdom x. Germany x. Australia x. Canada WHO - EDM

Essential information in report z. Patient information z. Adverse event or product problem z. Suspected medication z. Reporter 2/22/2021 WHO - EDM

Processing z. Reports are processed in batch z. Data mining techniques are used z. Technical/logical checks are made automatically y. Incorrect reports are rejected by the system and corrected manually WHO - EDM

Output z. Screening for signals y. By external review panel of experts z. Customised reports yon request by National Centres or pharmaceutical companies 2/22/2021 WHO - EDM

End Result z. Published in “Signal” document if verified z. Published in the Pharmaceutical Newsletter z. Change in product information 2/22/2021 WHO - EDM

Future challenges z. Raise awareness of the importance of monitoring all medicines z. Integrate work throughout all WHO z. Improve training activities at grass root level 2/22/2021 WHO - EDM

In conclusion …. z. The work of WHO in the area of safety monitoring of medicines is necessary if we are to achieve the mission of EDM: z. Medicines should be Available, Affordable, Safe and Properly used. 2/22/2021 WHO - EDM
 European directorate for the quality of medicines
European directorate for the quality of medicines Dr fiona couper yamba
Dr fiona couper yamba Bérénice se fait couper les cheveux
Bérénice se fait couper les cheveux Archibald scott couper
Archibald scott couper Archibald scott couper biografia
Archibald scott couper biografia Mary wollstonecraft mary a fiction
Mary wollstonecraft mary a fiction Army medical service corps officer career progression
Army medical service corps officer career progression Define primary health care
Define primary health care ükala
ükala Leeds grenville lanark population
Leeds grenville lanark population Veterinary medicines directorate
Veterinary medicines directorate Chapter 19 lesson 1 the role of medicines
Chapter 19 lesson 1 the role of medicines George's marvellous medicine story
George's marvellous medicine story Medicines management programme
Medicines management programme The drug basket method dispense medication to is used to
The drug basket method dispense medication to is used to Niyog niyogan uses and preparation
Niyog niyogan uses and preparation Summarize roosevelt's approach to environmental problems
Summarize roosevelt's approach to environmental problems Nhs dictionary of medicines and devices
Nhs dictionary of medicines and devices Medicines learning portal
Medicines learning portal Ectoparasiticides veterinary medicines
Ectoparasiticides veterinary medicines Abasagar
Abasagar Medicines complete
Medicines complete Cqc medicines management
Cqc medicines management Medicines information centre
Medicines information centre