Macitentan in Pulmonary Hypertension Due To Left Ventricular



- Slides: 8

Macitentan in Pulmonary Hypertension Due To Left Ventricular Dysfunction: MELODY-1 Jean-Luc Vachièry, Marion Delcroix, Hikmet Al-Hiti, Michela Efficace, Martin Hutyra, Gabriela Lack, Kelly Papadakis and Lewis J. Rubin Eur Respir J 2018; 51: 1701886

MELODY-1: Rationale • PH is a common in HF and is associated with significant morbidity and poor prognosis. • Recent guidelines defined two types of group 2 PH: isolated post capillary PH (Ipc. PH) and combined pre and post capillary PH (Cpc. PH). • In HF patients, endothelin-1 levels are elevated and associated with increased pulmonary pressures and higher mortality. • Macitentan is an endothelin-1 receptor antagonist (ETRA) approved for the long term treatment of PAH (group 1 PH). • Potential benefit of ETRAs has not been proven yet in HF. • Primary objective of Macitentan in Subjects with Combined pr. E and post Capi. Llary Pulm. Onary Hypertension due to Left Ventricular DYsfunction (MELODY-1) was to evaluate the safety and tolerability of macitentan in patients with Cpc. PH. Vachièry J-L, Delcroix M, Al-Hiti H, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J 2018; 51: 1701886

Methodology • Multicenter (28 sites), double-blind, randomized, placebo-controlled, phase II study. • Patients were included if were ≥ 18 years old with: • Confirmed Cpc. PH by RHC (mean PA ≥ 25 mm. Hg, PCWP 15 -25 mm. Hg, PVR ≥ 3 WU and DPG ≥ 7 mm. Hg) • EF ≥ 30% by echocardiogram • NYHA functional class II or III • 6 minute walk test ≥ 150 meters • Stable dose of diuretics • Patients were excluded if were taking prostacyclin analogues, phosphodiesterase type 5 inhibitors, guanylate cyclase or ERAs. • Randomization 1: 1 to macitentan 10 mg or placebo. • Primary safety end point: composite of significant fluid retention or worsening NYHA FC. • Increase in body weight by ≥ 5 % or 5 kg due to fluid overload • Use of parenteral diuretics • Selected secondary endpoints: • Change in hemodynamics Vachièry J-L, Delcroix M, Al-Hiti H, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J 2018; 51: 1701886 • Change in NT-pro. BNP

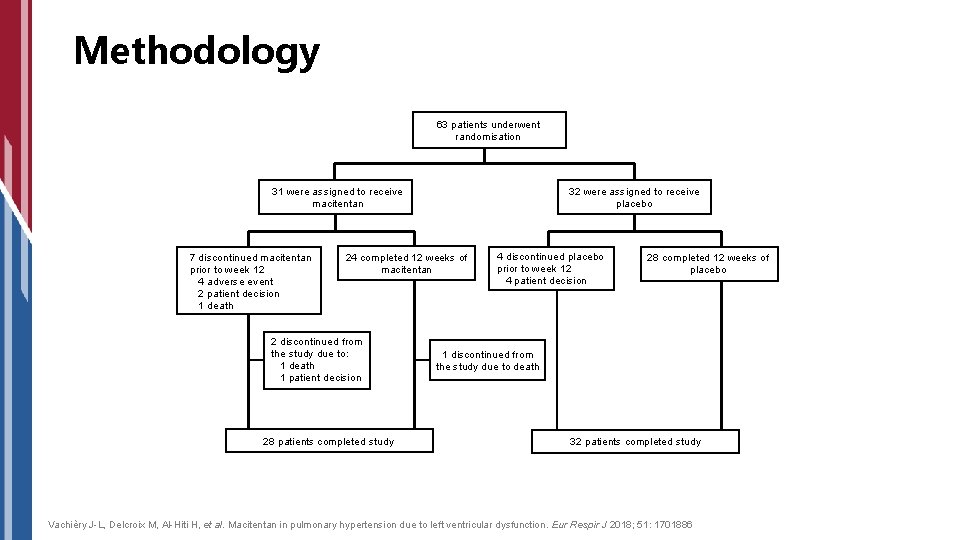
Methodology 63 patients underwent randomisation 31 were assigned to receive macitentan 7 discontinued macitentan prior to week 12 4 adverse event 2 patient decision 1 death 32 were assigned to receive placebo 24 completed 12 weeks of macitentan 2 discontinued from the study due to: 1 death 1 patient decision 28 patients completed study 4 discontinued placebo prior to week 12 4 patient decision 28 completed 12 weeks of placebo 1 discontinued from the study due to death 32 patients completed study Vachièry J-L, Delcroix M, Al-Hiti H, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J 2018; 51: 1701886

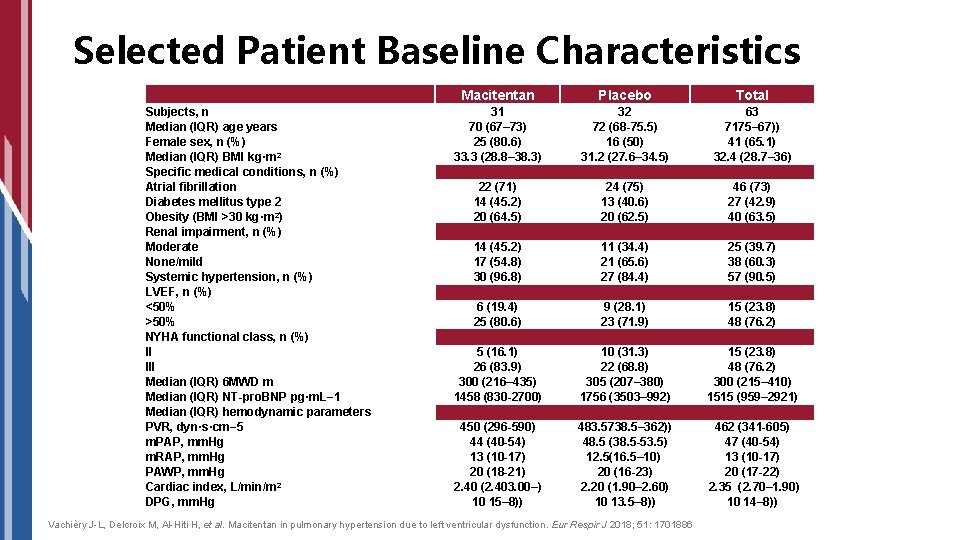
Selected Patient Baseline Characteristics Subjects, n Median (IQR) age years Female sex, n (%) Median (IQR) BMI kg·m 2 Specific medical conditions, n (%) Atrial fibrillation Diabetes mellitus type 2 Obesity (BMI >30 kg·m 2) Renal impairment, n (%) Moderate None/mild Systemic hypertension, n (%) LVEF, n (%) <50% >50% NYHA functional class, n (%) II III Median (IQR) 6 MWD m Median (IQR) NT-pro. BNP pg·m. L− 1 Median (IQR) hemodynamic parameters PVR, dyn·s·cm− 5 m. PAP, mm. Hg m. RAP, mm. Hg PAWP, mm. Hg Cardiac index, L/min/m 2 DPG, mm. Hg Macitentan Placebo Total 31 70 (67– 73) 25 (80. 6) 33. 3 (28. 8– 38. 3) 32 72 (68 -75. 5) 16 (50) 31. 2 (27. 6– 34. 5) 63 7175– 67)) 41 (65. 1) 32. 4 (28. 7– 36) 22 (71) 14 (45. 2) 20 (64. 5) 24 (75) 13 (40. 6) 20 (62. 5) 46 (73) 27 (42. 9) 40 (63. 5) 14 (45. 2) 17 (54. 8) 30 (96. 8) 11 (34. 4) 21 (65. 6) 27 (84. 4) 25 (39. 7) 38 (60. 3) 57 (90. 5) 6 (19. 4) 25 (80. 6) 9 (28. 1) 23 (71. 9) 15 (23. 8) 48 (76. 2) 5 (16. 1) 26 (83. 9) 300 (216– 435) 1458 (830 -2700) 10 (31. 3) 22 (68. 8) 305 (207– 380) 1756 (3503– 992) 15 (23. 8) 48 (76. 2) 300 (215– 410) 1515 (959– 2921) 450 (296 -590) 44 (40 -54) 13 (10 -17) 20 (18 -21) 2. 40 (2. 403. 00–) 10 15– 8)) 483. 5738. 5– 362)) 48. 5 (38. 5 -53. 5) 12. 5(16. 5– 10) 20 (16 -23) 2. 20 (1. 90– 2. 60) 10 13. 5– 8)) 462 (341 -605) 47 (40 -54) 13 (10 -17) 20 (17 -22) 2. 35 (2. 70– 1. 90) 10 14– 8)) Vachièry J-L, Delcroix M, Al-Hiti H, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J 2018; 51: 1701886

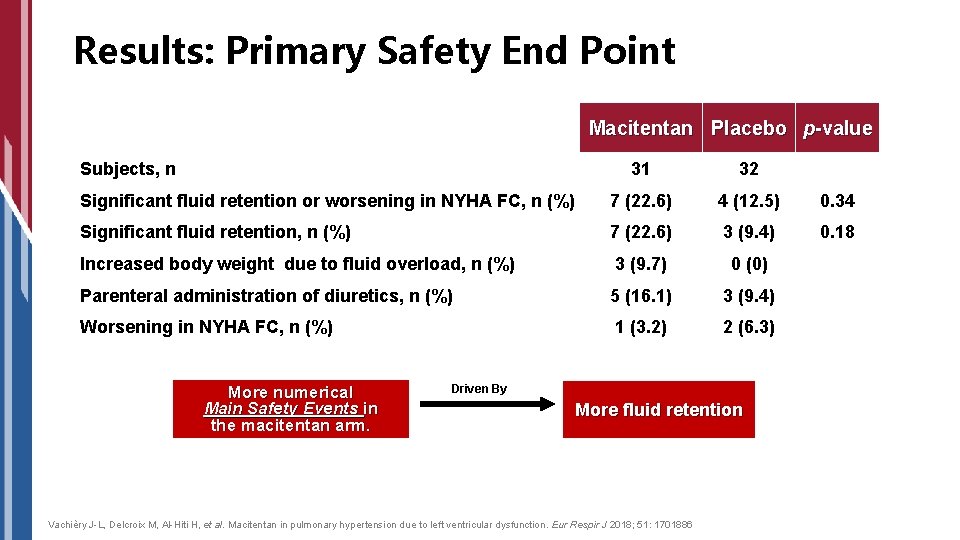
Results: Primary Safety End Point Macitentan Placebo p-value Subjects, n 31 32 Significant fluid retention or worsening in NYHA FC, n (%) 7 (22. 6) 4 (12. 5) 0. 34 Significant fluid retention, n (%) 7 (22. 6) 3 (9. 4) 0. 18 Increased body weight due to fluid overload, n (%) 3 (9. 7) 0 (0) Parenteral administration of diuretics, n (%) 5 (16. 1) 3 (9. 4) Worsening in NYHA FC, n (%) 1 (3. 2) 2 (6. 3) More numerical Main Safety Events in the macitentan arm. Driven By More fluid retention Vachièry J-L, Delcroix M, Al-Hiti H, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J 2018; 51: 1701886

Results: Other Secondary End Points No significant change in hemodynamics between groups Macitentan n Baseline, mean (SD) PVR, dyn∙sec/cm 5 20 525. 5 (270. 9) 357. 2 (195. 0) m. PAP, mm. Hg 21 (8. 7) 44. 6 m. RAP, mm. Hg 21 PAWP, mm. Hg Week 12, mean (SD) n Placebo Baseline, mean (SD) Week 12, mean (SD) 24 521. 4 (233. 9) 438. 7 (255. 4) 41. 1 (10. 7) 25 (10. 2) 45. 9 42. 1 (11. 2) 12. 1 (4. 6) 11. 2 (5. 7) 25 13. 0 (4. 7) 11. 3 (5. 2) 20 19. 1 (3. 6) (7) 19. 9 24 19. 7 (3. 6) (7) 20. 8 Cardiac index, L/min/m 2 20 2. 3 (0. 6) (0. 7) 2. 7 24 2. 3 (0. 6) DPG, mm. Hg 20 11. 8 (5. 9) 7. 0 (6. 1) 24 (4. 4) 11. 4 (7. 9) 7. 0 Reduction in NT-pro. BNP at 12 weeks in macitentan group Vachièry J-L, Delcroix M, Al-Hiti H, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J 2018; 51: 1701886

Summary and Conclusions • MELODY-1 was the first randomized controlled trial to evaluate the effect of ERA in Cpc. PH. • Overall, macitentan was associated with more fluid retention or worsening FC than placebo. • Interestingly, there was no change in RAP or PCWP, and there was a decrease in NT-pro. BNP in the patients assigned to macitentan. • In conclusion, in patients with Cpc. PH macitentan is associated with a numerically higher incidence of significant fluid retention compared with placebo despite no evidence of increased filling pressures. • Limitations: • Pilot study with a small study population and absence of formal power Vachièry J-L, Delcroix M, Al-Hiti H, et al. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J 2018; 51: 1701886 calculations.