PNEUMOTRIESTE 2011 Pulmonary hypertension and diffuse parenchymal diseases




























- Slides: 66

PNEUMOTRIESTE 2011 Pulmonary hypertension and diffuse parenchymal diseases: therapeutic options Carlo Albera Università di Torino Facoltà di Medicina e Chirurgia San Luigi Gonzaga Dipartimento di Scienze Cliniche e Biologiche Ambulatorio Interstiziopatie Polmonari / Malattie Rare

Pulmonary hypertension and diffuse parenchymal diseases: therapeutic options To date no approved drugs

Thank you for your attention

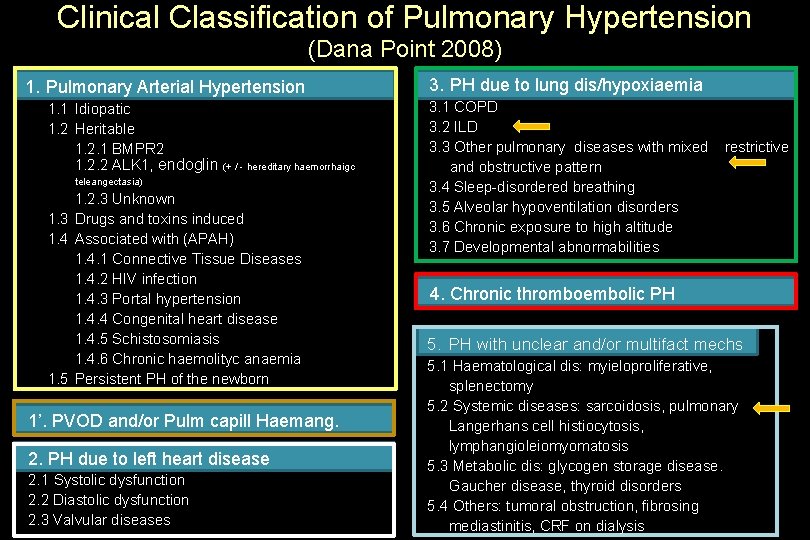
Clinical Classification of Pulmonary Hypertension (Dana Point 2008) 1. Pulmonary Arterial Hypertension 1. 1 Idiopatic 1. 2 Heritable 1. 2. 1 BMPR 2 1. 2. 2 ALK 1, endoglin (+ / - hereditary haemorrhaigc teleangectasia) 1. 2. 3 Unknown 1. 3 Drugs and toxins induced 1. 4 Associated with (APAH) 1. 4. 1 Connective Tissue Diseases 1. 4. 2 HIV infection 1. 4. 3 Portal hypertension 1. 4. 4 Congenital heart disease 1. 4. 5 Schistosomiasis 1. 4. 6 Chronic haemolityc anaemia 1. 5 Persistent PH of the newborn 1’. PVOD and/or Pulm capill Haemang. 2. PH due to left heart disease 2. 1 Systolic dysfunction 2. 2 Diastolic dysfunction 2. 3 Valvular diseases 3. PH due to lung dis/hypoxiaemia 3. 1 COPD 3. 2 ILD 3. 3 Other pulmonary diseases with mixed and obstructive pattern 3. 4 Sleep-disordered breathing 3. 5 Alveolar hypoventilation disorders 3. 6 Chronic exposure to high altitude 3. 7 Developmental abnormabilities restrictive 4. Chronic thromboembolic PH 5. PH with unclear and/or multifact mechs 5. 1 Haematological dis: myieloproliferative, splenectomy 5. 2 Systemic diseases: sarcoidosis, pulmonary Langerhans cell histiocytosis, lymphangioleiomyomatosis 5. 3 Metabolic dis: glycogen storage disease. Gaucher disease, thyroid disorders 5. 4 Others: tumoral obstruction, fibrosing mediastinitis, CRF on dialysis

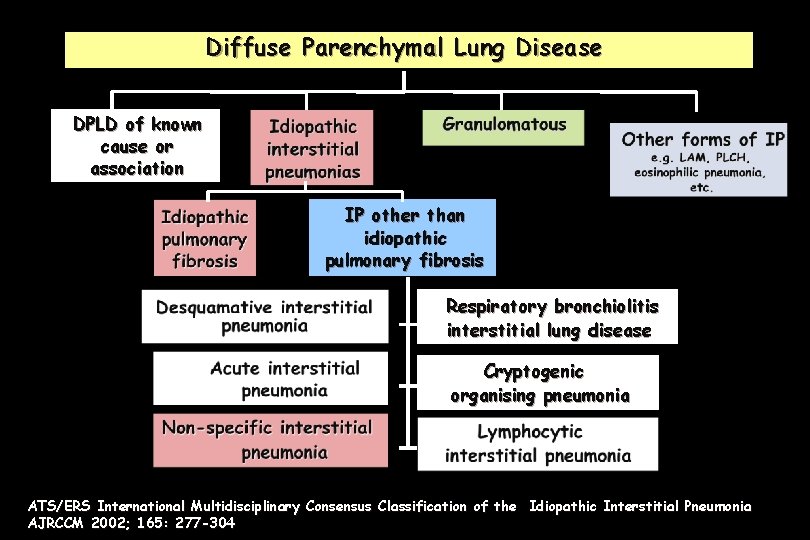
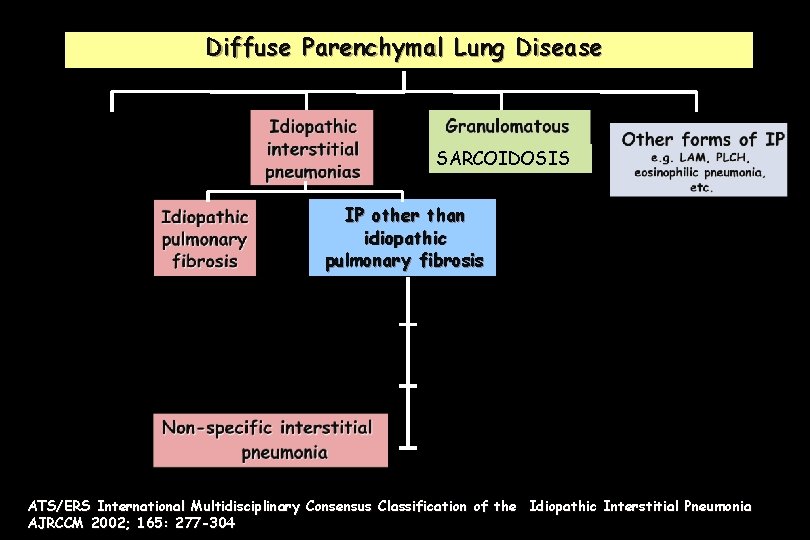
Diffuse Parenchymal Lung Disease DPLD of known cause or association IP other than idiopathic pulmonary fibrosis Respiratory bronchiolitis interstitial lung disease Cryptogenic organising pneumonia ATS/ERS International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonia AJRCCM 2002; 165: 277 -304

Pulmonary hypertension and ILDs What from guidelines ?

ATS/ERS/JRS/ALAT EBM GUIDELINES Am J Respir Crit Care Med 183, 788– 824, 2011

Treatment of selected complications and comorbid conditions There is an increasing awareness of complications and comorbid conditions frequently associated with IPF. These include acute exacerbation of IPF, pulmonary hypertension, gastroesophageal reflux disease, obesity, emphysema, and obstructive sleep apnea. It is unknown if treating these comorbidities influences clinical outcomes. ATS/ERS/JRS/ALAT EBM GUIDELINES Am J Respir Crit Care Med 183, 788– 824, 2011

Question: Should pulmonary hypertension be treated in patients with IPF ? (1) Recommendation: Pulmonary hypertension should not be treated in the majority of patients with IPF, but treatment may be a reasonable choice in a minority (weak recommendation, very low-quality evidence). Values: This recommendation places a high value on cost and the potential for drug-related morbidity, and a low value on very low-quality data suggesting a possible benefit in selected patients. ATS/ERS/JRS/ALAT EBM GUIDELINES Am J Respir Crit Care Med 183, 788– 824, 2011

Question: Should pulmonary hypertension be treated in patients with IPF ? (2) Remarks: In patients with moderate to severe pulmonary hypertension documented by right heart catheterization (i. e. , m. PAP ≥ 35 mm Hg), in line with the interpretation of a weak recommendation, a trial of vasomodulatory therapy may be indicated. The committee recognizes the need for clinical trials of vasomodulatory therapies in this patient population. (Vote: 8 for use, 14 against use, 1 abstention, 8 absent. ) ATS/ERS/JRS/ALAT EBM GUIDELINES Am J Respir Crit Care Med 183, 788– 824, 2011

Monitoring for Complications and Comorbidities: Comorbidities including pulmonary hypertension, pulmonary embolism, lung cancer, and coronary artery disease are known to occur in IPF. While the development of these comorbidities may influence survival, the role of routine screening to identify such complications in patients with IPF is unknown. (? ? ? ) Thus, a recommendation for routine screening cannot be made ATS/ERS/JRS/ALAT EBM GUIDELINES Am J Respir Crit Care Med 183, 788– 824, 2011

Monitoring for Complications and Comorbidities: pulmonary hypertension (1) In patients demonstrating progressive disease, the identification of PH may impact consideration for lung transplantation in eligible patients, and evaluation is indicated. Echocardiography is inaccurate in estimating pulmonary hemodynamics in patients with fibrotic lung disease and should not be relied upon to assess the presence and severity of pulmonary hypertension. ATS/ERS/JRS/ALAT EBM GUIDELINES Am J Respir Crit Care Med 183, 788– 824, 2011

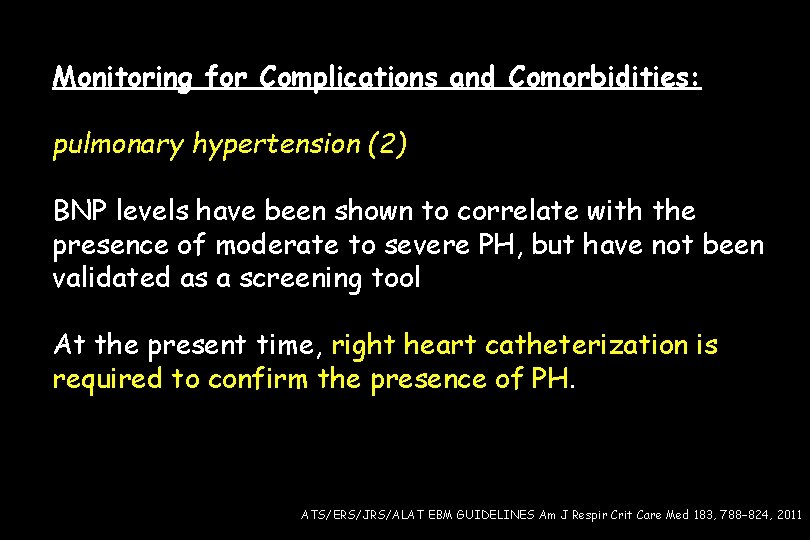
Monitoring for Complications and Comorbidities: pulmonary hypertension (2) BNP levels have been shown to correlate with the presence of moderate to severe PH, but have not been validated as a screening tool At the present time, right heart catheterization is required to confirm the presence of PH. ATS/ERS/JRS/ALAT EBM GUIDELINES Am J Respir Crit Care Med 183, 788– 824, 2011

European Heart Journal (2009) 30, 2493– 2537

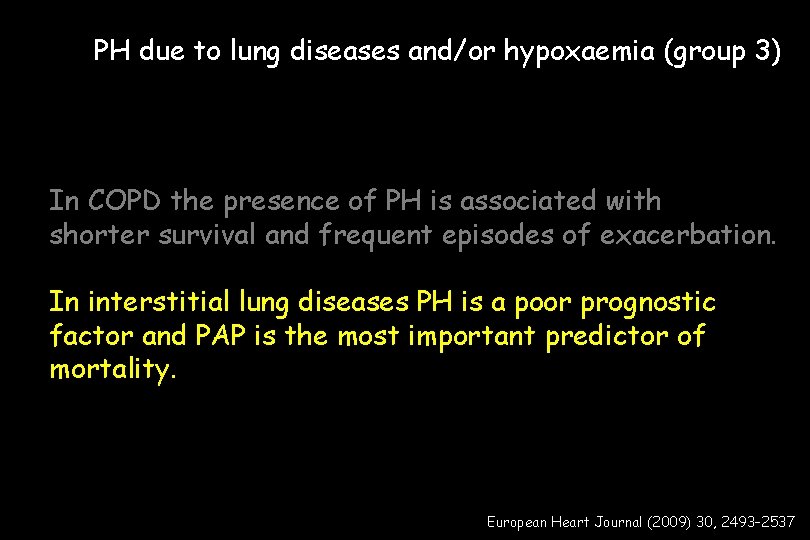
PH due to lung diseases and/or hypoxaemia (group 3) In COPD the presence of PH is associated with shorter survival and frequent episodes of exacerbation. In interstitial lung diseases PH is a poor prognostic factor and PAP is the most important predictor of mortality. European Heart Journal (2009) 30, 2493– 2537

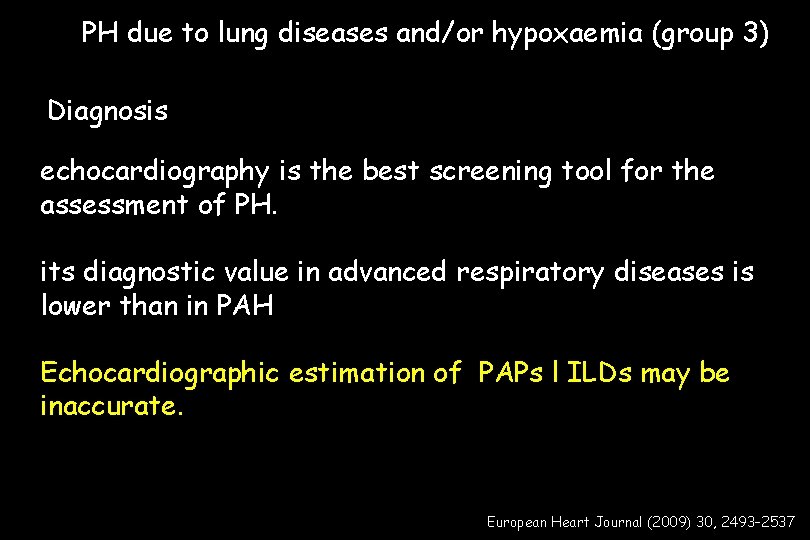
PH due to lung diseases and/or hypoxaemia (group 3) Diagnosis echocardiography is the best screening tool for the assessment of PH. its diagnostic value in advanced respiratory diseases is lower than in PAH Echocardiographic estimation of PAPs l ILDs may be inaccurate. European Heart Journal (2009) 30, 2493– 2537

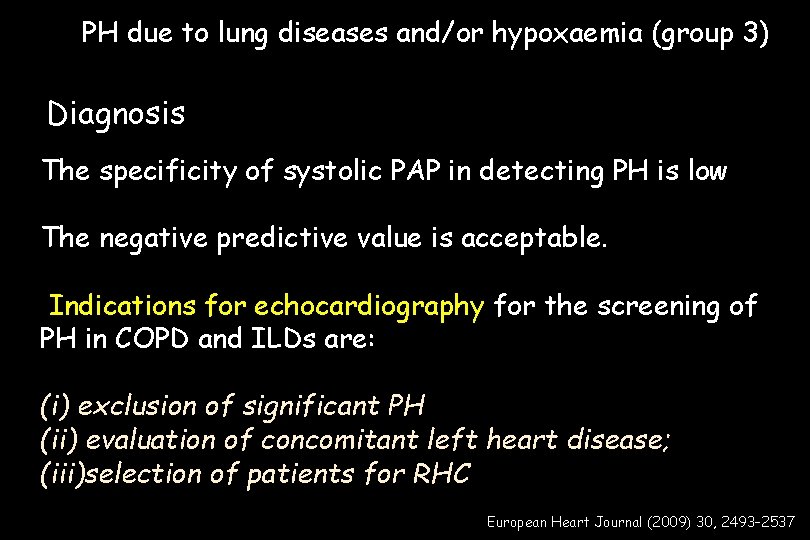
PH due to lung diseases and/or hypoxaemia (group 3) Diagnosis The specificity of systolic PAP in detecting PH is low The negative predictive value is acceptable. Indications for echocardiography for the screening of PH in COPD and ILDs are: (i) exclusion of significant PH (ii) evaluation of concomitant left heart disease; (iii)selection of patients for RHC European Heart Journal (2009) 30, 2493– 2537

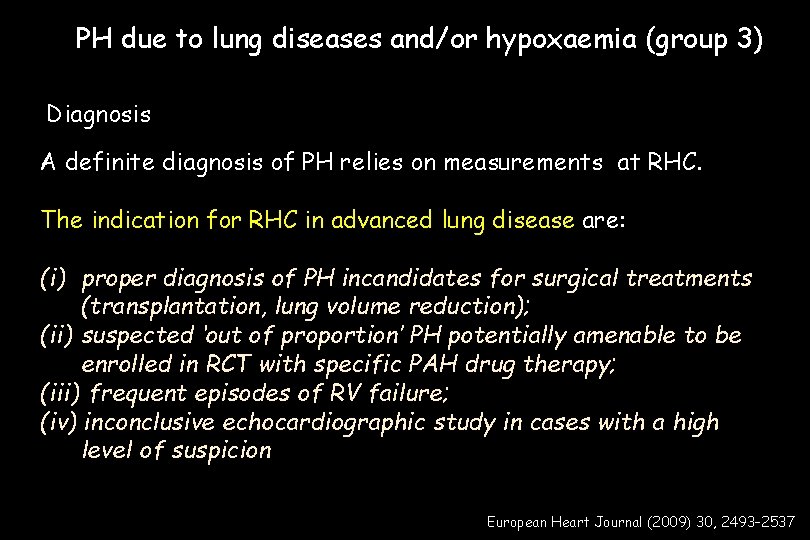
PH due to lung diseases and/or hypoxaemia (group 3) Diagnosis A definite diagnosis of PH relies on measurements at RHC. The indication for RHC in advanced lung disease are: (i) proper diagnosis of PH incandidates for surgical treatments (transplantation, lung volume reduction); (ii) suspected ‘out of proportion’ PH potentially amenable to be enrolled in RCT with specific PAH drug therapy; (iii) frequent episodes of RV failure; (iv) inconclusive echocardiographic study in cases with a high level of suspicion European Heart Journal (2009) 30, 2493– 2537

PH due to lung diseases and/or hypoxaemia (group 3) Therapy Currently there is no specific therapy for PH associated with COPD or interstitial lung diseases. Long-term O 2 administration has been shown partially toreduce the progression of PH in COPD. Nevertheless, with this treatment PAP rarely returns to normal values and the structural abnormalities of pulmonary vessels remain unaltered. In interstitial lung diseases, the role of long-term O 2 therapy in PH progression is less clear. European Heart Journal (2009) 30, 2493– 2537

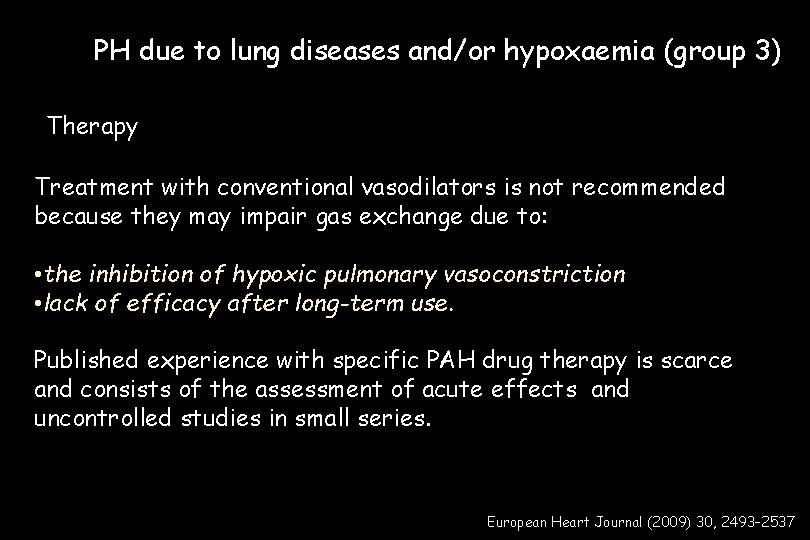
PH due to lung diseases and/or hypoxaemia (group 3) Therapy Treatment with conventional vasodilators is not recommended because they may impair gas exchange due to: • the inhibition of hypoxic pulmonary vasoconstriction • lack of efficacy after long-term use. Published experience with specific PAH drug therapy is scarce and consists of the assessment of acute effects and uncontrolled studies in small series. European Heart Journal (2009) 30, 2493– 2537

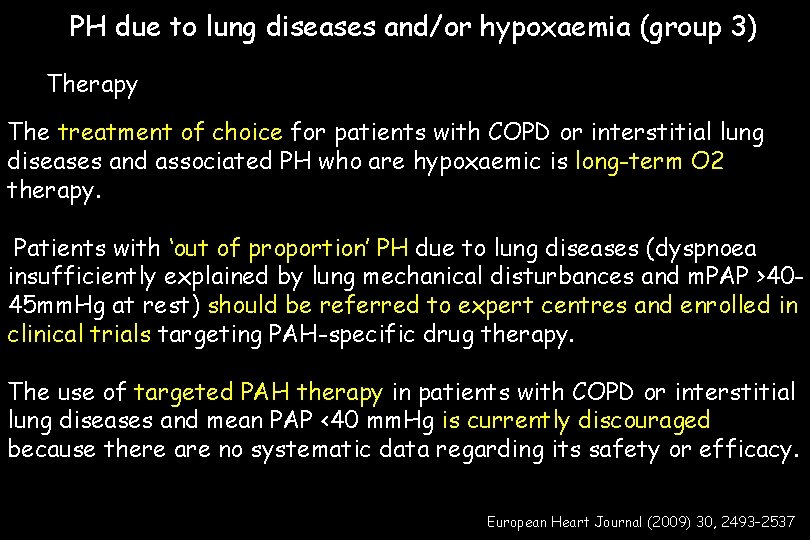
PH due to lung diseases and/or hypoxaemia (group 3) Therapy The treatment of choice for patients with COPD or interstitial lung diseases and associated PH who are hypoxaemic is long-term O 2 therapy. Patients with ‘out of proportion’ PH due to lung diseases (dyspnoea insufficiently explained by lung mechanical disturbances and m. PAP >4045 mm. Hg at rest) should be referred to expert centres and enrolled in clinical trials targeting PAH-specific drug therapy. The use of targeted PAH therapy in patients with COPD or interstitial lung diseases and mean PAP <40 mm. Hg is currently discouraged because there are no systematic data regarding its safety or efficacy. European Heart Journal (2009) 30, 2493– 2537

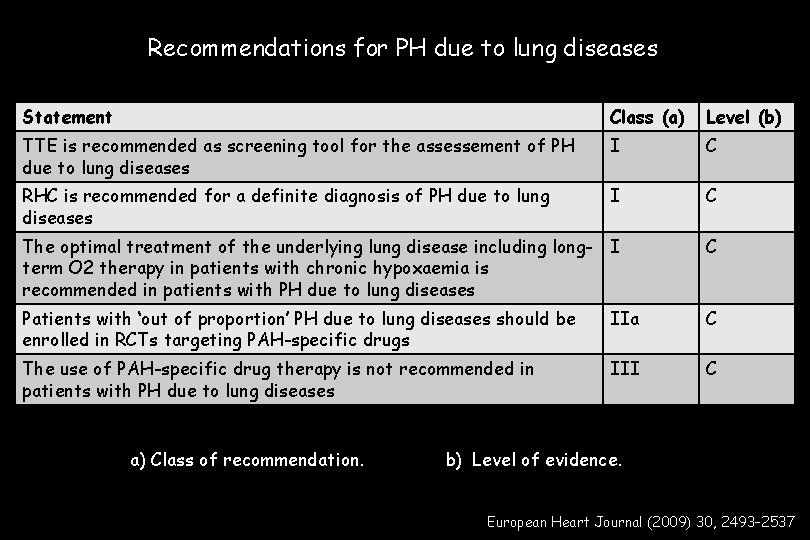
Recommendations for PH due to lung diseases Statement Class (a) Level (b) TTE is recommended as screening tool for the assessement of PH due to lung diseases I C RHC is recommended for a definite diagnosis of PH due to lung diseases I C The optimal treatment of the underlying lung disease including long- I term O 2 therapy in patients with chronic hypoxaemia is recommended in patients with PH due to lung diseases C Patients with ‘out of proportion’ PH due to lung diseases should be enrolled in RCTs targeting PAH-specific drugs IIa C The use of PAH-specific drug therapy is not recommended in patients with PH due to lung diseases III C a) Class of recommendation. b) Level of evidence. European Heart Journal (2009) 30, 2493– 2537

Take home message from guidelines Actually there are no EBM-supported therapeutic options for the treatment of PH associated to ILDs The therapy of ILDs is probably the best way to prevent/delay the development of associated PH Effective antifibrotic drug(s) may offer in the next future some new chances The treatment of ILDs-associated PH represent a still unmet medical need ILDs- associated PH if “Out proportion” may be considered and managed in a different way

Some additional data on ILDs and PH

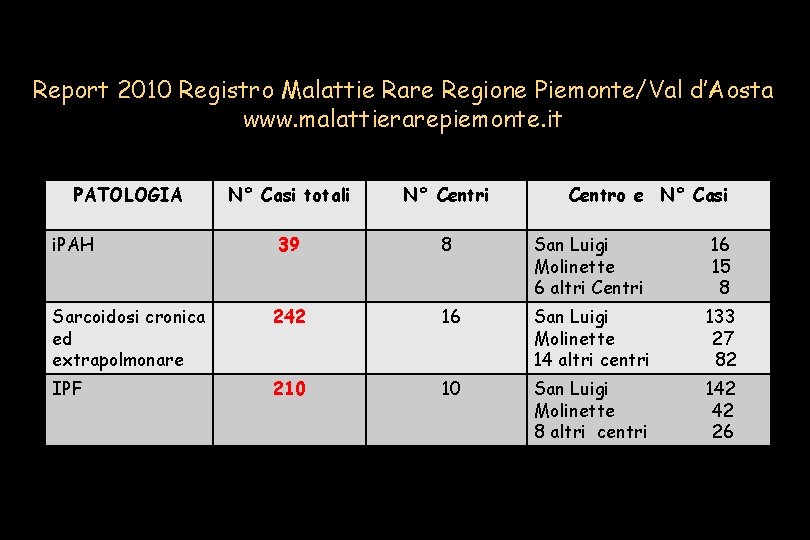
Report 2010 Registro Malattie Rare Regione Piemonte/Val d’Aosta www. malattierarepiemonte. it PATOLOGIA N° Casi totali N° Centri 39 8 San Luigi Molinette 6 altri Centri 16 15 8 Sarcoidosi cronica ed extrapolmonare 242 16 San Luigi Molinette 14 altri centri 133 27 82 IPF 210 10 San Luigi Molinette 8 altri centri 142 42 26 i. PAH Centro e N° Casi

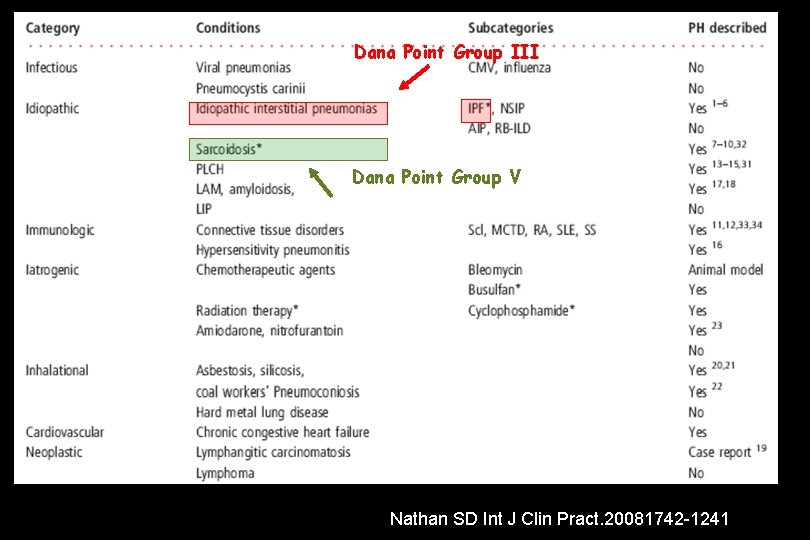
Dana Point Group III Dana Point Group V Nathan SD Int J Clin Pract. 20081742 -1241

Diffuse Parenchymal Lung Disease SARCOIDOSIS IP other than idiopathic pulmonary fibrosis ATS/ERS International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonia AJRCCM 2002; 165: 277 -304

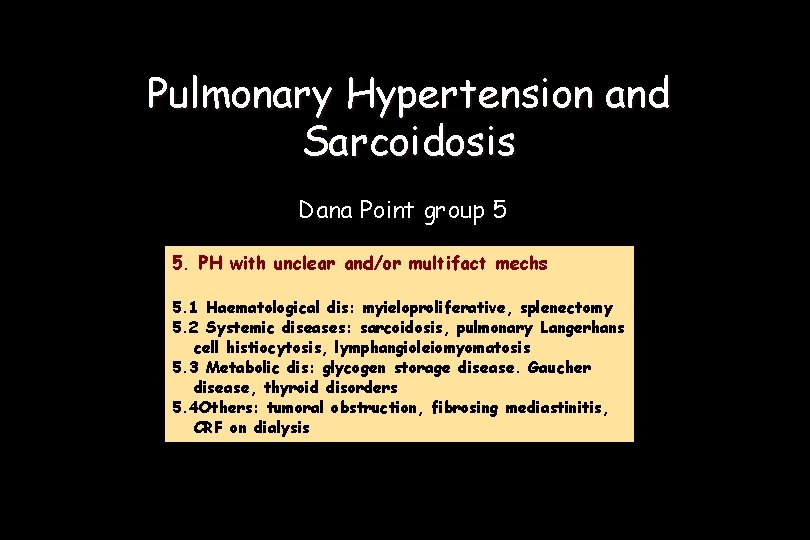
Pulmonary Hypertension and Sarcoidosis Dana Point group 5 5. PH with unclear and/or multifact mechs 5. 1 Haematological dis: myieloproliferative, splenectomy 5. 2 Systemic diseases: sarcoidosis, pulmonary Langerhans cell histiocytosis, lymphangioleiomyomatosis 5. 3 Metabolic dis: glycogen storage disease. Gaucher disease, thyroid disorders 5. 4 Others: tumoral obstruction, fibrosing mediastinitis, CRF on dialysis

Prospective, observational study 246 consecutive sarcoidosis patients PH defined as estimated s. PAP > 40 mm Hg 212 patients evaluated for s. PAP 12 patients (5. 7%) had PH. Decreased lung volume increases the risk of PH developing in patients with sarcoidosis. Handa, T et al. CHEST 2006; 129: 1246– 1252

Arthritis Research & Therapy 2007, 9(Suppl 2): S 8

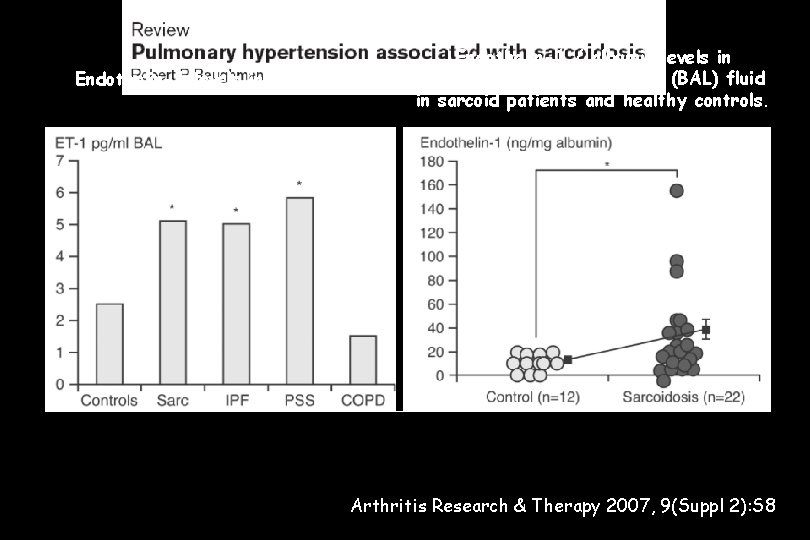
Endothelin-1 levels in BAL fluid Endothelin-1 / albumin levels in bronchoalveolar lavage fluid (BAL) fluid in sarcoid patients and healthy controls. Arthritis Research & Therapy 2007, 9(Suppl 2): S 8

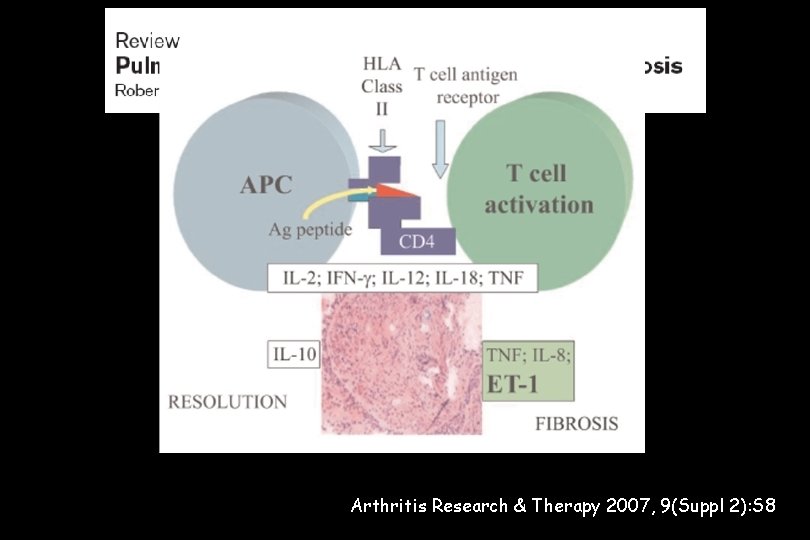
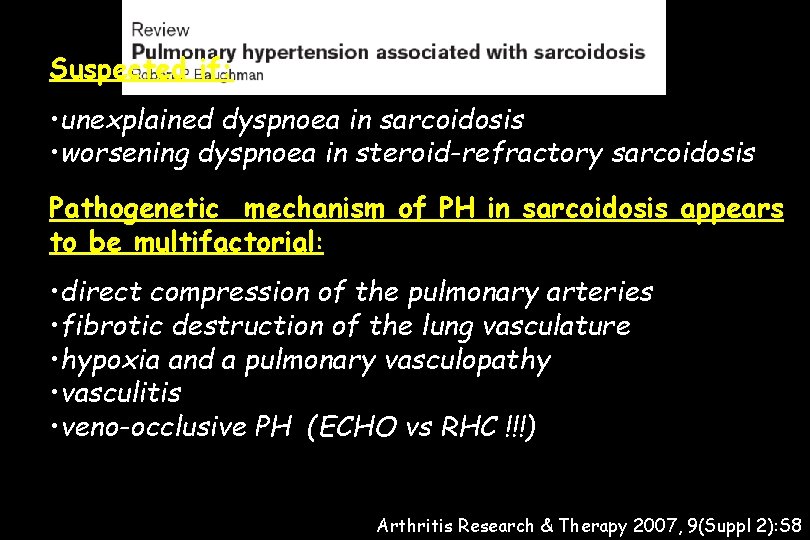
Suspected if: • unexplained dyspnoea in sarcoidosis • worsening dyspnoea in steroid-refractory sarcoidosis Pathogenetic mechanism of PH in sarcoidosis appears to be multifactorial: • direct compression of the pulmonary arteries • fibrotic destruction of the lung vasculature • hypoxia and a pulmonary vasculopathy • vasculitis • veno-occlusive PH (ECHO vs RHC !!!) Arthritis Research & Therapy 2007, 9(Suppl 2): S 8

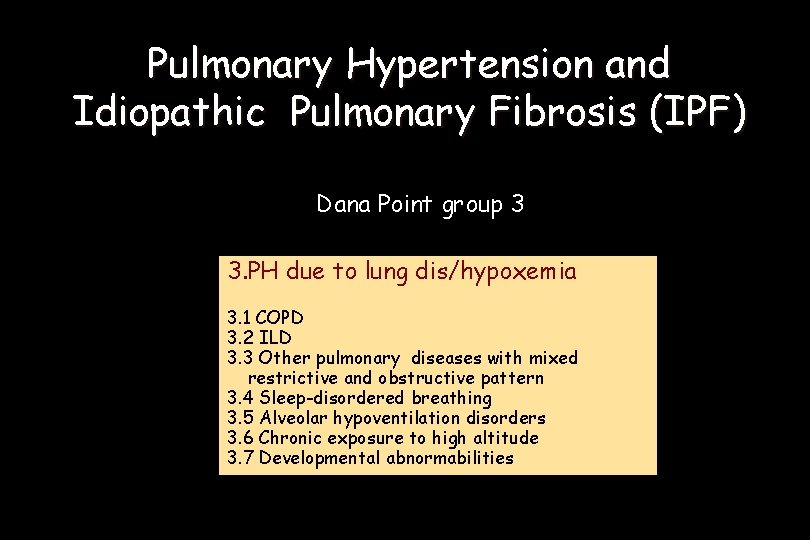
Pulmonary Hypertension and Idiopathic Pulmonary Fibrosis (IPF) Dana Point group 3 3. PH due to lung dis/hypoxemia 3. 1 COPD 3. 2 ILD 3. 3 Other pulmonary diseases with mixed restrictive and obstructive pattern 3. 4 Sleep-disordered breathing 3. 5 Alveolar hypoventilation disorders 3. 6 Chronic exposure to high altitude 3. 7 Developmental abnormabilities

Pulmonary Hypertension in IPF • Pathogenesis • Prevalence, detection, diagnosis • Course and significance • Therapy

Pulmonary Hypertension in IPF • Pathogenesis • Prevalence, detection, diagnosis • Course and significance • Therapy

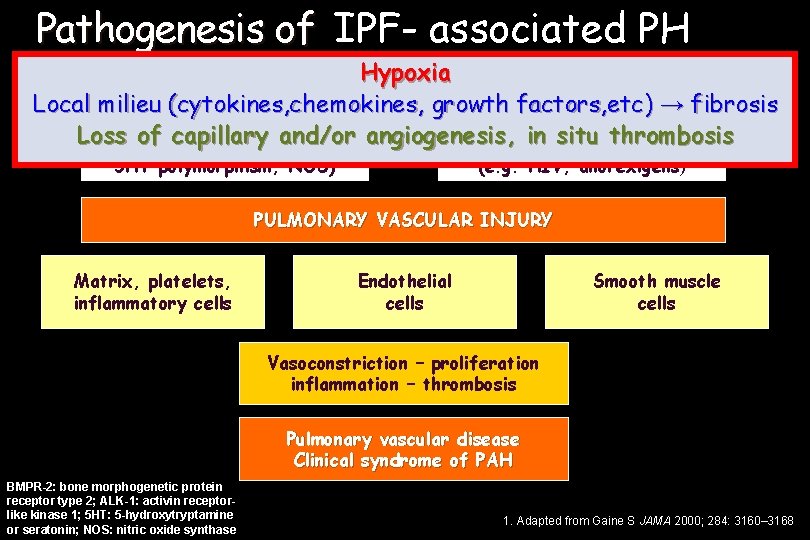
Pathogenesis of PAH IPF- associated PH Hypoxia Local milieu (cytokines, chemokines, growth factors, etc) → fibrosis Genetic predisposition Risk factors and Loss of capillary and/or angiogenesis , in situconditions thrombosis (mutations BMPR-2, ALK-1, associated 5 HT polymorphism, NOS) (e. g. HIV, anorexigens) PULMONARY VASCULAR INJURY Matrix, platelets, inflammatory cells Endothelial cells Smooth muscle cells Vasoconstriction – proliferation inflammation – thrombosis Pulmonary vascular disease Clinical syndrome of PAH BMPR-2: bone morphogenetic protein receptor type 2; ALK-1: activin receptorlike kinase 1; 5 HT: 5 -hydroxytryptamine or seratonin; NOS: nitric oxide synthase 1. Adapted from Gaine S JAMA 2000; 284: 3160– 3168

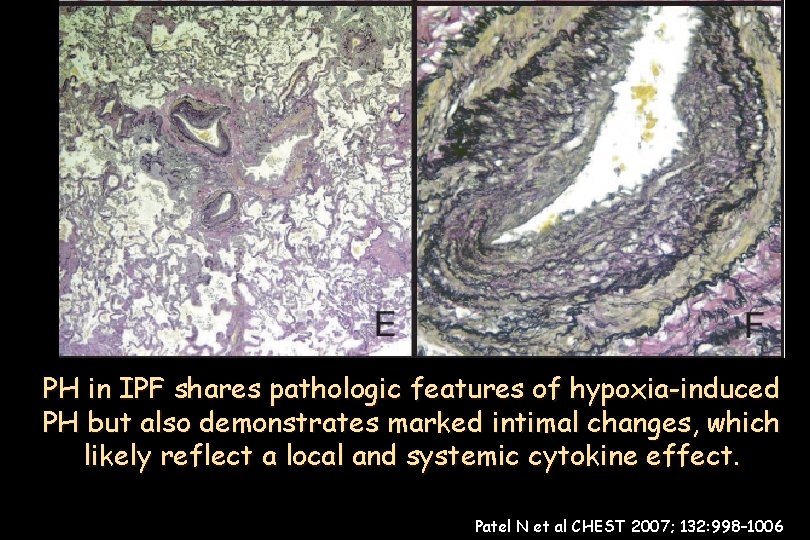
(n 376) bronchiole-vascular bundle in in aanlobule area medial +medial intimalthickening +elastic tissue Pulmonary artery/arteriole marked PH in IPF shares pathologic features of hypoxia-induced Pulmonary artery branch inlung an area of Medial thickening and significant intimal ofrelatively nonfibrotic tissue duplication of nonfibrotic lung tissue. dense fibrosis. proliferation. . PH but also demonstrates marked intimal changes, which likely reflect a local and systemic cytokine effect. Patel N et al CHEST 2007; 132: 998– 1006

Pulmonary Hypertension in IPF • Pathogenesis • Prevalence, detection, diagnosis • Course and significance • Therapy

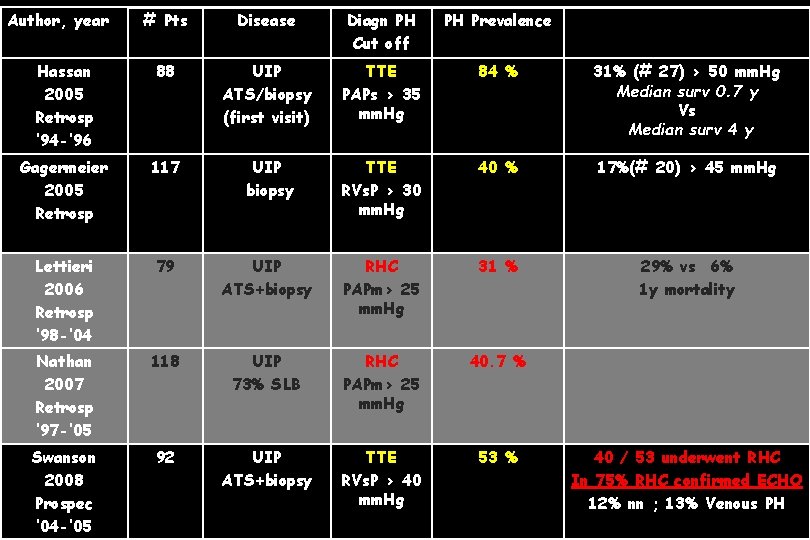
Author, year # Pts Disease Diagn PH Cut off PH Prevalence Hassan 2005 Retrosp ’ 94 -’ 96 88 UIP ATS/biopsy (first visit) TTE PAPs > 35 mm. Hg 84 % 31% (# 27) > 50 mm. Hg Median surv 0. 7 y Vs Median surv 4 y Gagermeier 2005 Retrosp 117 UIP biopsy TTE RVs. P > 30 mm. Hg 40 % 17%(# 20) > 45 mm. Hg Lettieri 2006 Retrosp ’ 98 -’ 04 79 UIP ATS+biopsy RHC PAPm> 25 mm. Hg 31 % 29% vs 6% 1 y mortality Nathan 2007 Retrosp ‘ 97 -’ 05 118 UIP 73% SLB RHC PAPm> 25 mm. Hg 40. 7 % Swanson 2008 Prospec ‘ 04 -’ 05 92 UIP ATS+biopsy TTE RVs. P > 40 mm. Hg 53 % 40 / 53 underwent RHC In 75% RHC confirmed ECHO 12% nn ; 13% Venous PH

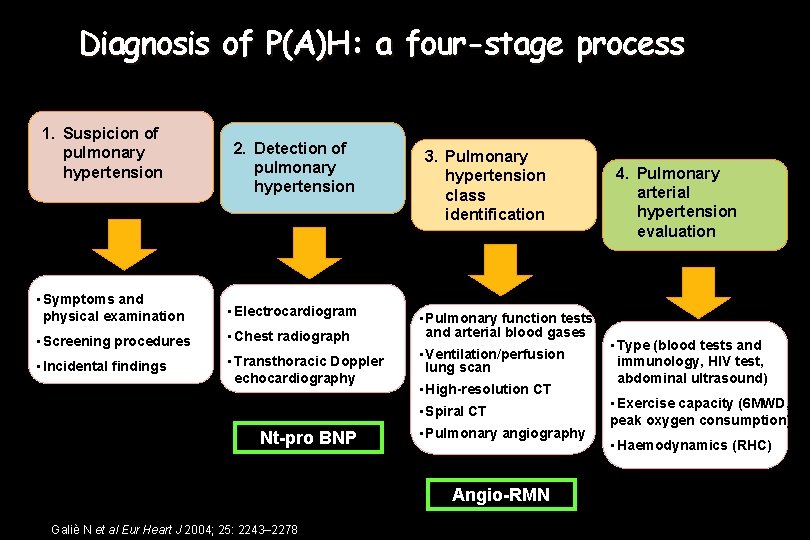
Diagnosis of P(A)H: a four-stage process 1. Suspicion of pulmonary hypertension 2. Detection of pulmonary hypertension • Symptoms and physical examination • Electrocardiogram • Screening procedures • Chest radiograph • Incidental findings • Transthoracic Doppler echocardiography 3. Pulmonary hypertension class identification • Pulmonary function tests and arterial blood gases • Ventilation/perfusion lung scan • High-resolution CT • Spiral CT Nt-pro BNP • Pulmonary angiography Angio-RMN Galiè N et al Eur Heart J 2004; 25: 2243– 2278 4. Pulmonary arterial hypertension evaluation • Type (blood tests and immunology, HIV test, abdominal ultrasound) • Exercise capacity (6 MWD, peak oxygen consumption) • Haemodynamics (RHC)

Pulmonary Hypertension in IPF • Pathogenesis • Prevalence, detection, diagnosis • Course and significance • Therapy

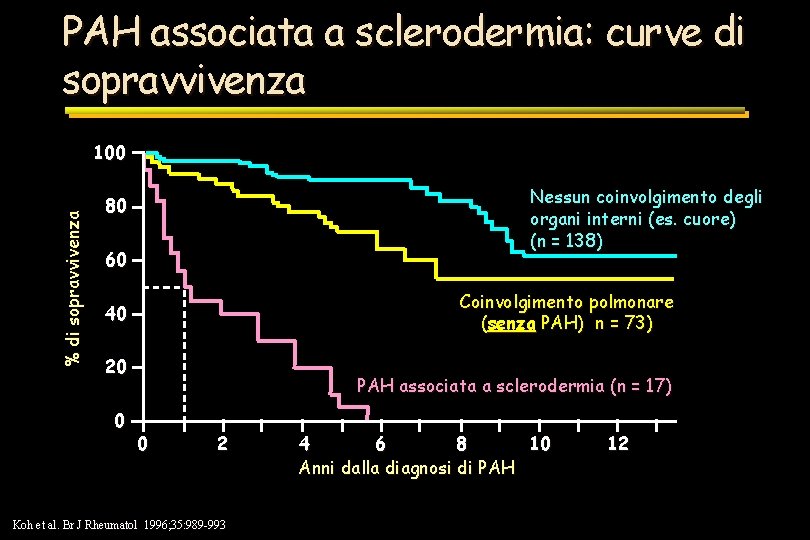
PAH associata a sclerodermia: curve di sopravvivenza % di sopravvivenza 100 Nessun coinvolgimento degli organi interni (es. cuore) (n = 138) 80 60 Coinvolgimento polmonare (senza PAH) n = 73) 40 20 0 PAH associata a sclerodermia (n = 17) 0 2 Koh et al. Br J Rheumatol 1996; 35: 989 -993 4 6 8 10 Anni dalla diagnosi di PAH 12

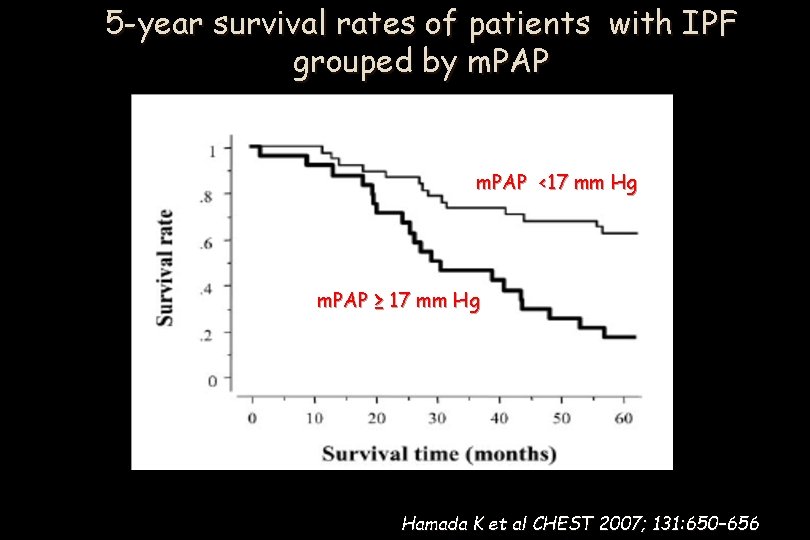
5 -year survival rates of patients with IPF grouped by m. PAP <17 mm Hg m. PAP ≥ 17 mm Hg Hamada K et al CHEST 2007; 131: 650– 656

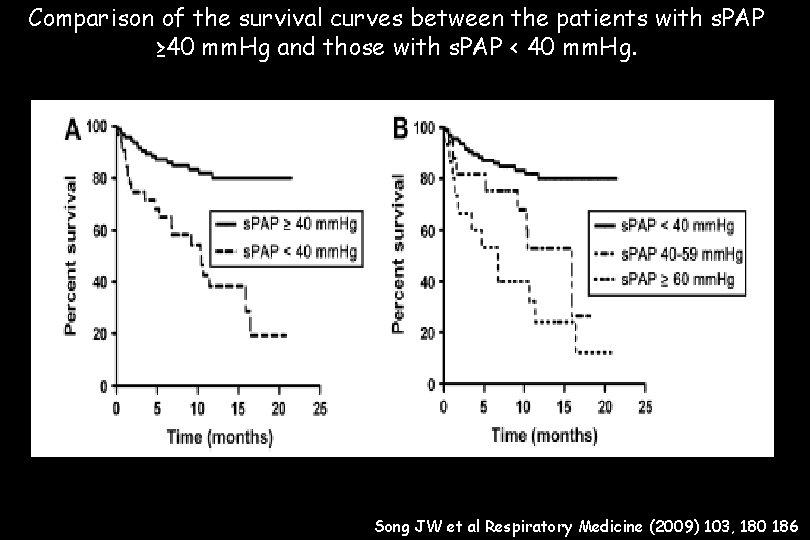
Comparison of the survival curves between the patients with s. PAP ≥ 40 mm. Hg and those with s. PAP < 40 mm. Hg. Song JW et al Respiratory Medicine (2009) 103, 180 186

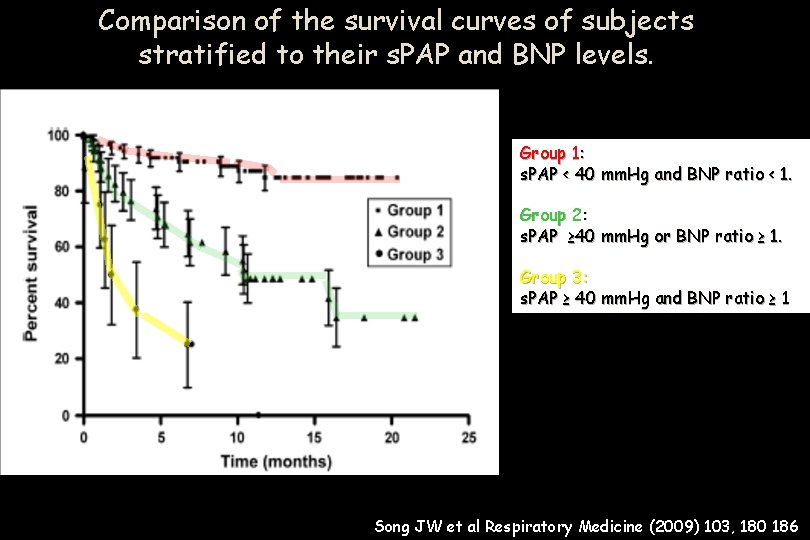
Comparison of the survival curves of subjects stratified to their s. PAP and BNP levels. Group 1: s. PAP < 40 mm. Hg and BNP ratio < 1. Group 2: s. PAP ≥ 40 mm. Hg or BNP ratio ≥ 1. Group 3: s. PAP ≥ 40 mm. Hg and BNP ratio ≥ 1 Song JW et al Respiratory Medicine (2009) 103, 180 186

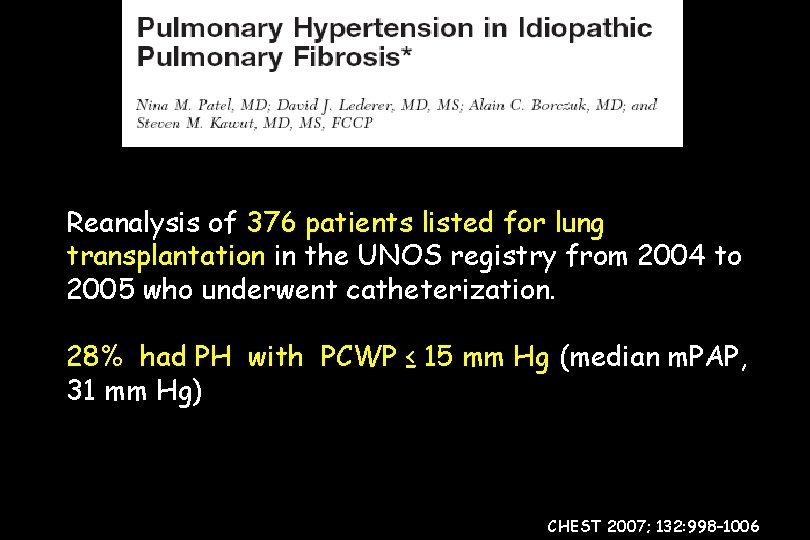
Reanalysis of 376 patients listed for lung transplantation in the UNOS registry from 2004 to 2005 who underwent catheterization. 28% had PH with PCWP ≤ 15 mm Hg (median m. PAP, 31 mm Hg) CHEST 2007; 132: 998– 1006

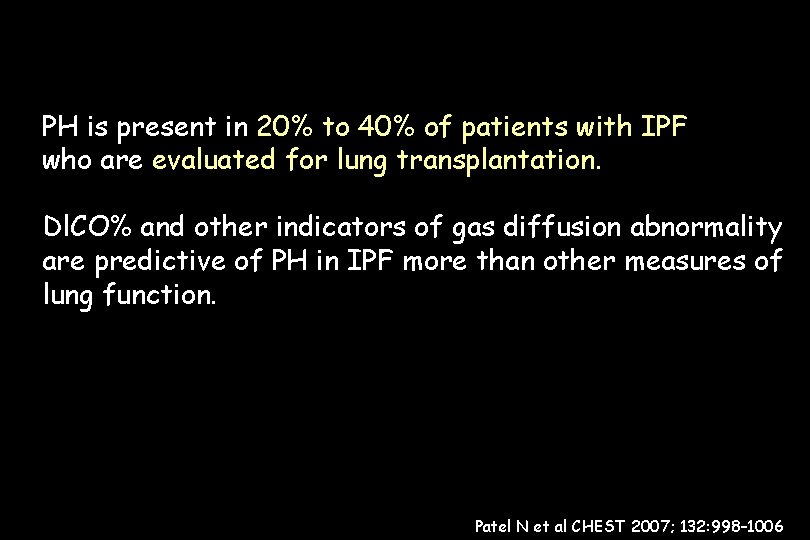
PH is present in 20% to 40% of patients with IPF (n 376) who are evaluated for lung transplantation. Dl. CO% and other indicators of gas diffusion abnormality are predictive of PH in IPF more than other measures of lung function. Patel N et al CHEST 2007; 132: 998– 1006

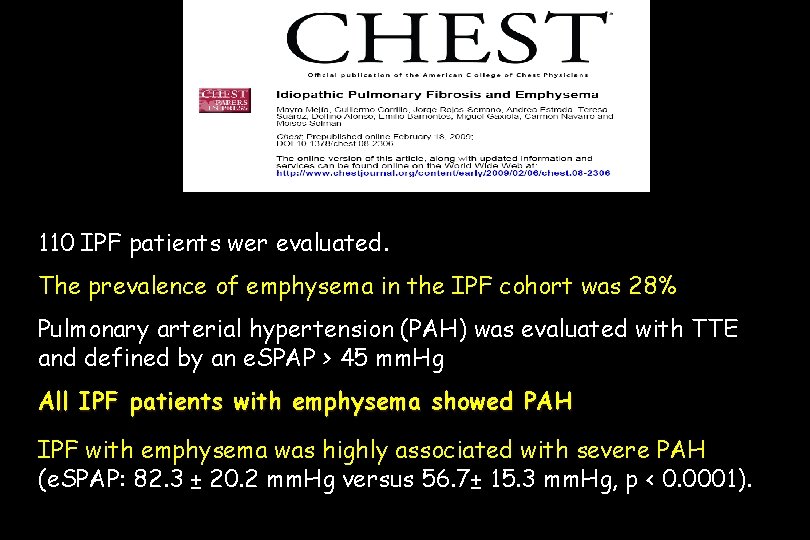
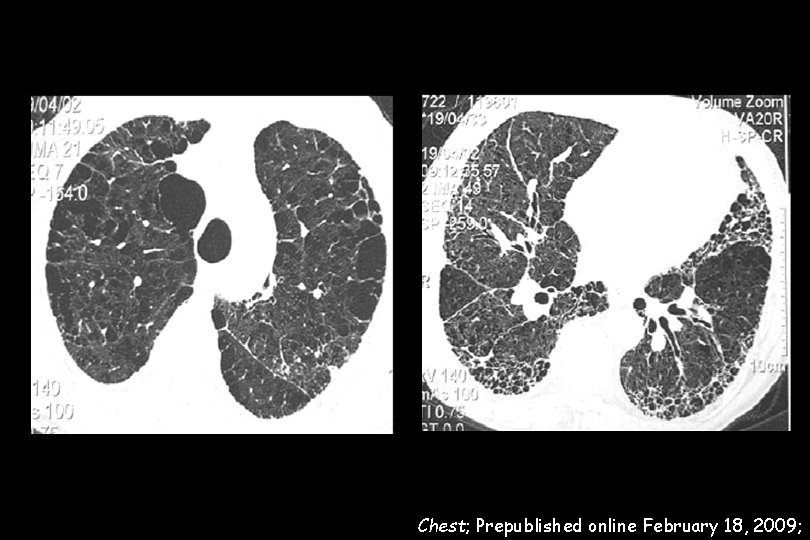
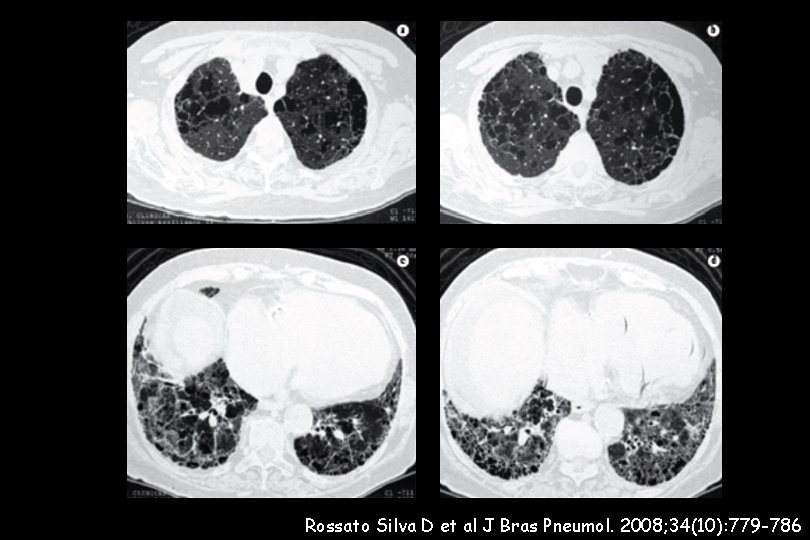
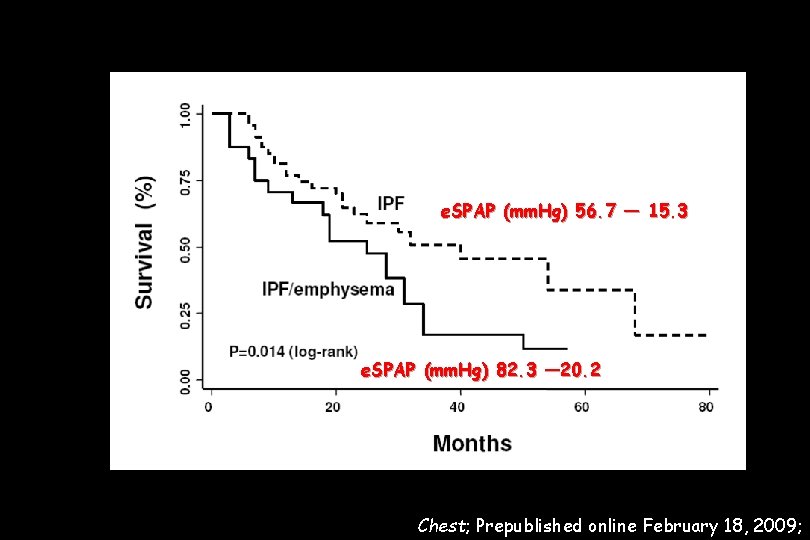
110 IPF patients wer evaluated. The prevalence of emphysema in the IPF cohort was 28% Pulmonary arterial hypertension (PAH) was evaluated with TTE and defined by an e. SPAP > 45 mm. Hg All IPF patients with emphysema showed PAH IPF with emphysema was highly associated with severe PAH (e. SPAP: 82. 3 ± 20. 2 mm. Hg versus 56. 7± 15. 3 mm. Hg, p < 0. 0001).

Chest; Prepublished online February 18, 2009;

Rossato Silva D et al J Bras Pneumol. 2008; 34(10): 779 -786

e. SPAP (mm. Hg) 56. 7 ― 15. 3 e. SPAP (mm. Hg) 82. 3 ― 20. 2 Chest; Prepublished online February 18, 2009;

“Out of proportion” PH in IPF Advanced PH in the presence of underlying lung disease of less severity raises the possibility of a primary vasculopathy that is at least partly independent from the underlying parenchymal process. (Girgis, Clin Chest Med, 2007) Based on analogy to primary pulmonary hypertension, more specific interventions aiming at the restoration of endothelial vasoconstrictor-dilator imbalance could be undertaken. Randomized controlled trials of oral therapy endothelin receptor blockers and phosphodiesterase 5 inhibitors are being considered. (Naeije, Proc Am Thorac Soc, 2005)

Pulmonary Hypertension in IPF • Pathogenesis • Prevalence, detection, diagnosis • Course and significance • Therapy

Treatment of ph in the context of ILD : A still unmet need The primary treatment approach is directed to control the underlying ILD Do we have any active drug for IPF ? Treatment recommendations for patients with PH have only been established for PAH patients, which refers to Group I patients of the Dana Point classification No clear evidence of that the same approach may be useful in all cases of ILD-associated. Off PH label drugs: careful and limited use !! ECHO and to be included in. ILD the isdiagnostic workout Screening for PHRHC in each patient with mandatory and follow-up in IPF ? RCS are needed RCS : in progress; study design: end-points !!

CHEST 2006; 130: 182– 189

Immunosuppressive Therapy in Connective Tissue Diseases-Associated PAH No patients with SSC associated PAH did respond PAH associated with SLE or MCTD might respond to a treatment combining glucocorticosteroids and cyclophosphamide. CHEST 2006; 130: 182– 189

57

Work in progress

ARTEMIS-PH - Study of Ambrisentan in Subjects With Pulmonary Hypertension Associated With Idiopathic Pulmonary Fibrosis (Phase III) X This study will compare the efficacy and safety of ambrisentan to placebo in subjects with pulmonary hypertension associated with idiopathic pulmonary fibrosis.

Double Blind, Randomized Trial of Bosentan for Sarcoidosis Associated Pulmonary Hypertension (BOSAPAH) (Phase II-III) Patients with advanced sarcoidosis often develop pulmonary hypertension. The purpose of this study is to determine if bosentan (Tracleer) will help sarcoidosis associated pulmonary hypertension.

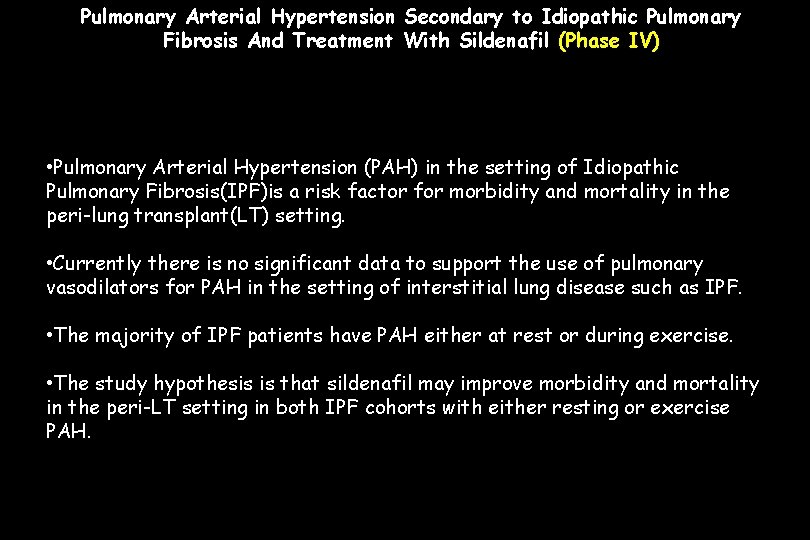
Pulmonary Arterial Hypertension Secondary to Idiopathic Pulmonary Fibrosis And Treatment With Sildenafil (Phase IV) • Pulmonary Arterial Hypertension (PAH) in the setting of Idiopathic Pulmonary Fibrosis(IPF)is a risk factor for morbidity and mortality in the peri-lung transplant(LT) setting. • Currently there is no significant data to support the use of pulmonary vasodilators for PAH in the setting of interstitial lung disease such as IPF. • The majority of IPF patients have PAH either at rest or during exercise. • The study hypothesis is that sildenafil may improve morbidity and mortality in the peri-LT setting in both IPF cohorts with either resting or exercise PAH.

A controlled trial of sildenafil in advanced IPF 80 patients enrolled in the study. Primary outcome : • proportion of patients with an increase in the 6 -minute walk distance of 20% or more. Key secondary outcomes: • changes in oxygenation • degree of dyspnea • quality of life The difference in the primary outcome was not significant Zisman DA, et al N Engl J Med. 2010 12; 363(7): 620 -8.

There were small but significant differences in key secondary endpoints: arterial oxygenation Dl. CO degree of dyspnea quality of life favoring the sildenafil group. Serious adverse events were similar in the two study groups. This study did not show a benefit for sildenafil for the primary outcome. The presence of some positive secondary outcomes creates clinical equipoise for further research. Zisman DA, et al N Engl J Med. 2010 12; 363(7): 620 -8.

Treprostinil Therapy For Patients With Interstitial Lung Disease And Severe Pulmonary Arterial Hypertension (Phase III) Using Either Intravenous (IV) or Subcutaneous (SQ) Treprostinil to Treat Pulmonary Hypertension Related to Underlying Interstitial Lung Disease The hypothesis is that IV or SQ Treprostinil can improve 6 minute walk distance, hemodynamics and quality of life in patients with interstitial lung disease and severe secondary pulmonary arterial hypertension.

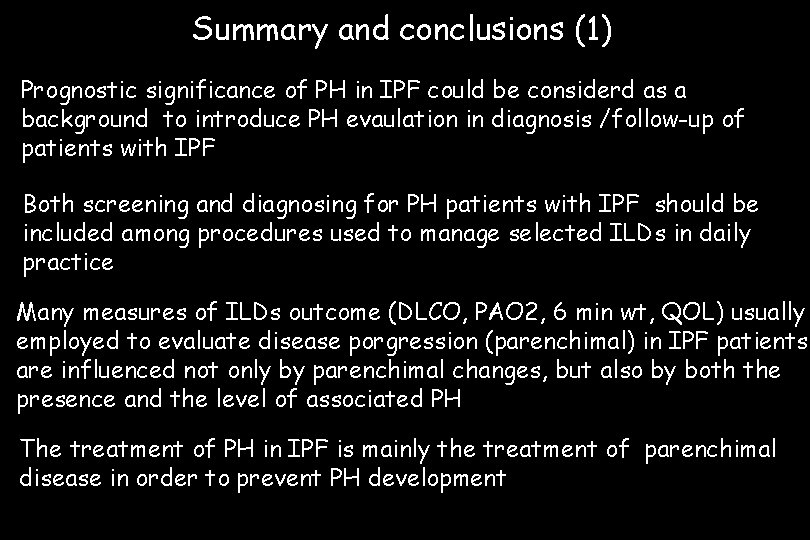
Summary and conclusions (1) Prognostic significance of PH in IPF could be considerd as a background to introduce PH evaulation in diagnosis /follow-up of patients with IPF Both screening and diagnosing for PH patients with IPF should be included among procedures used to manage selected ILDs in daily practice Many measures of ILDs outcome (DLCO, PAO 2, 6 min wt, QOL) usually employed to evaluate disease porgression (parenchimal) in IPF patients are influenced not only by parenchimal changes, but also by both the presence and the level of associated PH The treatment of PH in IPF is mainly the treatment of parenchimal disease in order to prevent PH development

Summary and conclusions (2) When PH develops lesson learned from previous experiences in treating PAH must be taken in account (in PAH associated with PSS no drug approved for patients with PAH associated to UIP-like parenchymal disease) Treatment of PH associated to ILDs is a still unmet need and even in presence of negative results of RC trials further studies are recruiting patients Early detection, diagnosis of PH in IPF probably will play a not negligible role in our daily practice for the clinical management of patients with UIP
 Medslide
Medslide Pulmonary hypertension severity
Pulmonary hypertension severity Pulmonary hypertension differential diagnosis
Pulmonary hypertension differential diagnosis Pulmonary hypertension definition
Pulmonary hypertension definition Bmpr gene
Bmpr gene Mitral stenosis pulmonary hypertension
Mitral stenosis pulmonary hypertension Dianne zwicke
Dianne zwicke Chd pulmonary hypertension
Chd pulmonary hypertension Pulmonary hypertension
Pulmonary hypertension Stages of pulmonary hypertension
Stages of pulmonary hypertension Pulmonary hypertension
Pulmonary hypertension Pathophysiology of pulmonary hypertension
Pathophysiology of pulmonary hypertension Decapneizzatore
Decapneizzatore Pneumotrieste
Pneumotrieste Pneumotrieste
Pneumotrieste Pneumotrieste
Pneumotrieste Focus mode and diffuse mode
Focus mode and diffuse mode Effuse and diffuse
Effuse and diffuse Portal hypertension signs
Portal hypertension signs Vray light lister
Vray light lister Diffuse sozialbeziehung oevermann
Diffuse sozialbeziehung oevermann Sclerenchyma
Sclerenchyma Grisaille diffuse
Grisaille diffuse Diffuse roughness
Diffuse roughness Plano convex lens
Plano convex lens Ungerichtete reflexion
Ungerichtete reflexion Varus esophagus
Varus esophagus Religioni più diffuse
Religioni più diffuse How does popular culture diffuse
How does popular culture diffuse Diffuse interstellar bands
Diffuse interstellar bands How does folk culture diffuse
How does folk culture diffuse Linguistic diversity ap human geography definition
Linguistic diversity ap human geography definition Explain, using kinetic particle theory, why gases diffuse.
Explain, using kinetic particle theory, why gases diffuse. Diffuse interstellar bands
Diffuse interstellar bands Laryngitis
Laryngitis Ambient diffuse specular
Ambient diffuse specular Kerley b lines
Kerley b lines Qcrack
Qcrack Hydrosonografie
Hydrosonografie Diffuse axonal injury
Diffuse axonal injury Diffuse peritonitis
Diffuse peritonitis Dendritic keratitis
Dendritic keratitis Mesothelioma
Mesothelioma Diffuse interstellar bands
Diffuse interstellar bands Serous diffuse myocarditis
Serous diffuse myocarditis Regular vs diffuse reflection
Regular vs diffuse reflection Diffuse rendering
Diffuse rendering At stp which gas will diffuse more readily than ne
At stp which gas will diffuse more readily than ne Hypertension artérielle
Hypertension artérielle Vital signs cpr
Vital signs cpr Hypertension
Hypertension Jnc7
Jnc7 Pachyméninges
Pachyméninges Hypertension
Hypertension Equivalence hbpm
Equivalence hbpm Hypertension
Hypertension Htn emergency vs urgency
Htn emergency vs urgency Demadex
Demadex Varices cardio tubérositaires
Varices cardio tubérositaires Non pharmacological management of hypertension
Non pharmacological management of hypertension Rules of halves in hypertension
Rules of halves in hypertension Hypertension medical definition
Hypertension medical definition Definition of hypertension
Definition of hypertension Masked hypertension
Masked hypertension Causes of secondary hypertension
Causes of secondary hypertension Features of cld
Features of cld Hypertension vs hypotension
Hypertension vs hypotension