LOGO Anusorn Ditsawan 51312333 Silapakorn University Confirmation of











- Slides: 36

LOGO Anusorn Ditsawan 51312333 Silapakorn University

Confirmation of Amphetamine , Methamphetamine , MDA and MDMA in urine samples using disk solid-phase extraction and gas chromatography–mass spectrometry after immunoassay screening Anusorn Ditsawan

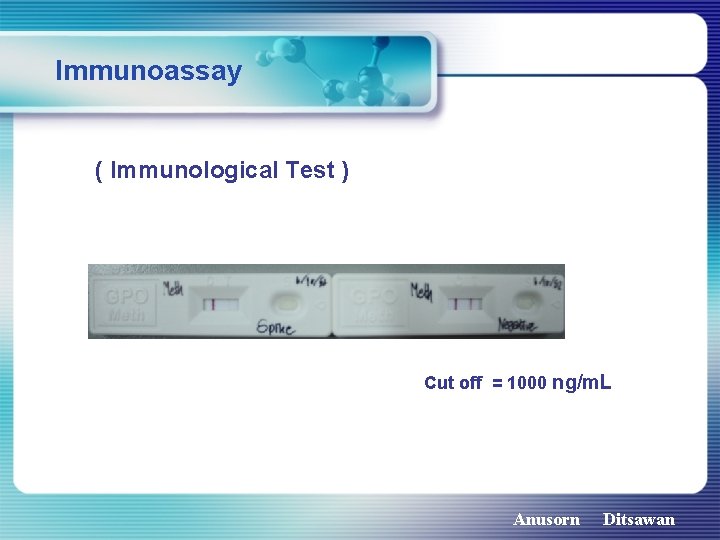
Immunoassay ( Immunological Test ) Cut off = 1000 ng/m. L Anusorn Ditsawan

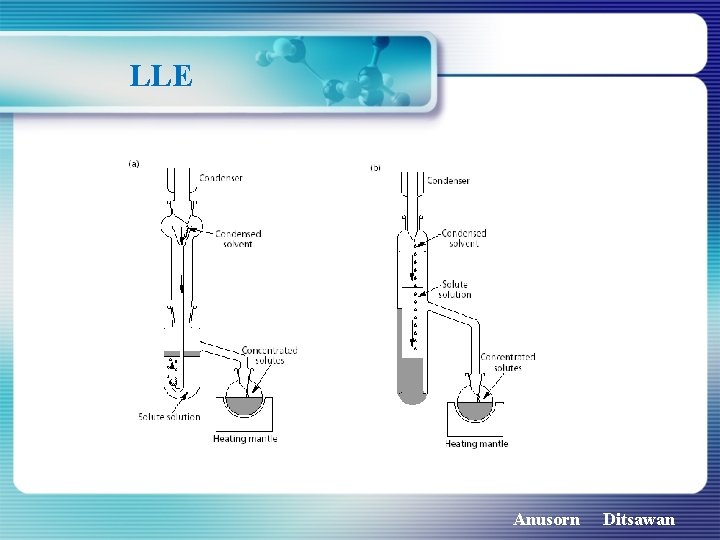
LLE Anusorn Ditsawan

GC-MS Agilent 6890 GC instrument An 7683 autosampler A 5973 N mass-selective detector GC-MS

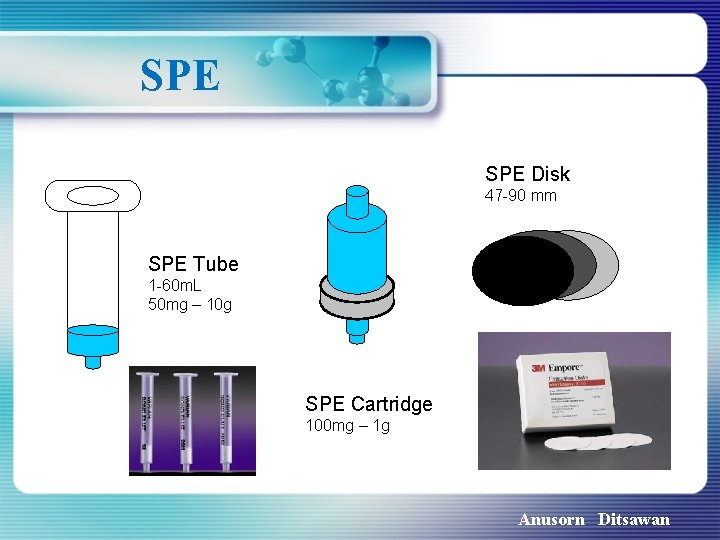
SPE Disk 47 -90 mm SPE Tube 1 -60 m. L 50 mg – 10 g SPE Cartridge 100 mg – 1 g Anusorn Ditsawan

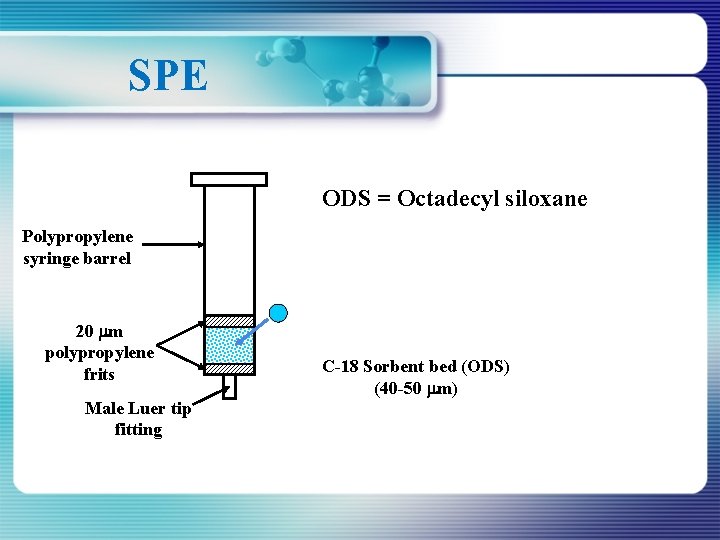
SPE ODS = Octadecyl siloxane Polypropylene syringe barrel 20 m polypropylene frits Male Luer tip fitting C-18 Sorbent bed (ODS) (40 -50 m)

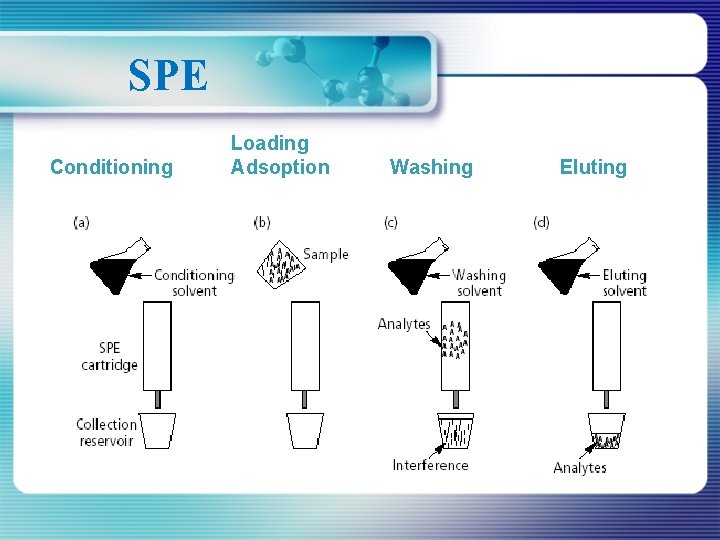
SPE Conditioning Loading Adsoption Washing Eluting

Anusorn Ditsawan

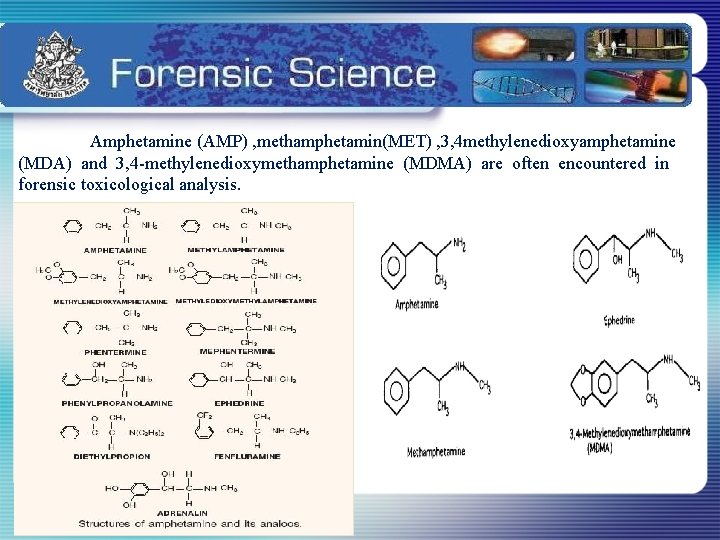
Introduction Amphetamines are powerful central nervous system (CNS) stimulants Chronic abuse of amphetamines causes hallucinations and psychosis , in addition to dysphoria and depression upon withdrawal

Amphetamine (AMP) , methamphetamin(MET) , 3, 4 methylenedioxyamphetamine (MDA) and 3, 4 -methylenedioxymethamphetamine (MDMA) are often encountered in forensic toxicological analysis.

Using SPE with strong cation-exchange (SCX) interaction, most of neutral and acidic impurities are washed away in the washing step, therefore, cleaner extract can be obtained. The investigation described here used the SPEC. PLUS. C 18 AR/MP 3 disk SPE cartridges to extract the amphetamines from urine samples. GC–MS working in the selected ion monitoring (SIM) mode was employed for confirmation and quantitation.

Experimental Amphetamine standards of reagent grade Ø Ø Amphetamine sulfate methamphetamine hydrochloride MDA hydrochloride MDMA hydrochloride Stock solutions of 1 mg/ml each drug (infreebases) were prepared separately by diluting appropriate amounts of the amphetamine standards (insaltforms), respectively , inproper volumes of methanol. Anusorn Ditsawan

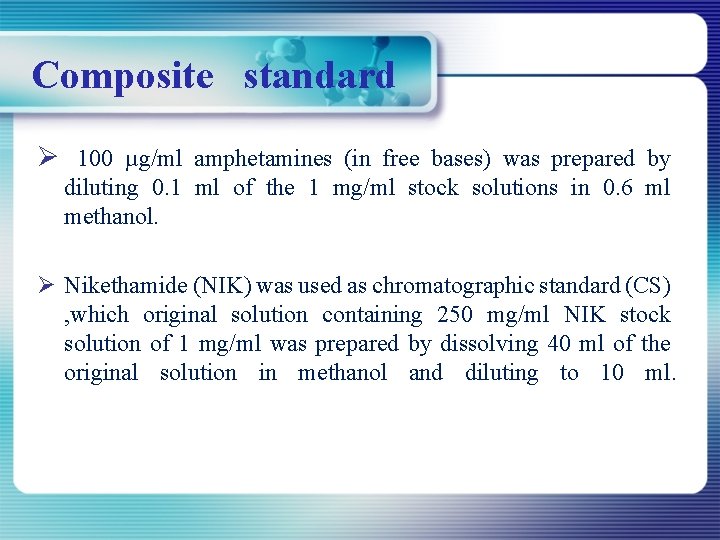
Composite standard Ø 100 µg/ml amphetamines (in free bases) was prepared by diluting 0. 1 ml of the 1 mg/ml stock solutions in 0. 6 ml methanol. Ø Nikethamide (NIK) was used as chromatographic standard (CS) , which original solution containing 250 mg/ml NIK stock solution of 1 mg/ml was prepared by dissolving 40 ml of the original solution in methanol and diluting to 10 ml.

Ø A working solution of 100 µg/ml NIK was prepared by diluting 0. 1 ml of the stock solution in 0. 9 ml methanol , and 10 ug/ml of working solution was prepared by diluting 0. 1 ml of the 100 µg/ml working solution in 0. 9 ml methanol Ø Phosphate buffer (p. H 6. 0) of 0. 1 M was prepared by dissolving 15. 6 g sodium dihydrogenphosphate in distilled water , diluting to 1000 ml and adjusting to the specific p. H with sodium hydrogenphosphate

Ø Carbonate buffer (p. H 10. 3) of 0. 1 M was prepared by dissolving 10. 6 g sodium carbonate (Na. CO) and 8. 4 g sodium hydrogencarbonate (Na. HCO) in distilled water and diluting to 1000 ml.

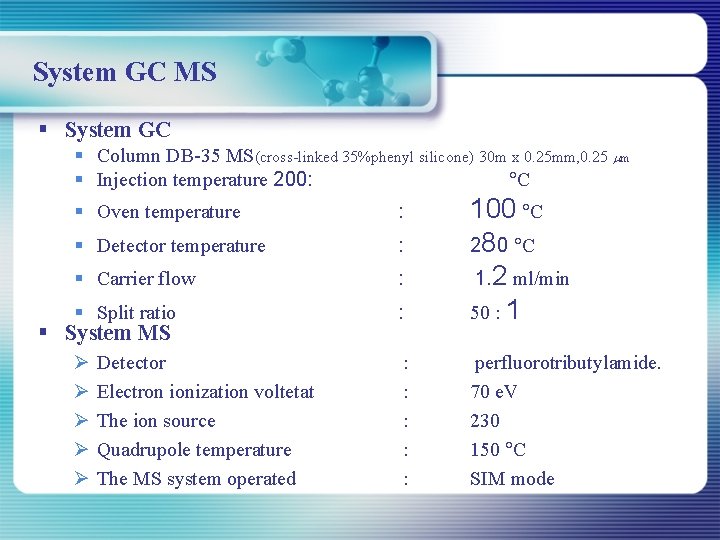
System GC MS § System GC § Column DB-35 MS(cross-linked 35%phenyl silicone) 30 m x 0. 25 mm, 0. 25 m § Injection temperature 200: C § Oven temperature : § Detector temperature : § Carrier flow : § Split ratio : 100 C 280 C 1. 2 ml/min 50 : 1 Ø Ø Ø : : : perfluorotributylamide. 70 e. V 230 150 C SIM mode § System MS Detector Electron ionization voltetat The ion source Quadrupole temperature The MS system operated

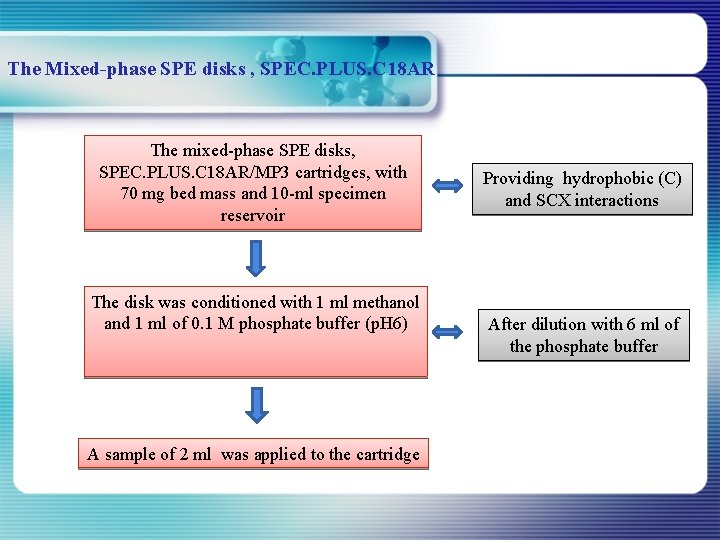
The Mixed-phase SPE disks , SPEC. PLUS. C 18 AR The mixed-phase SPE disks, SPEC. PLUS. C 18 AR/MP 3 cartridges, with 70 mg bed mass and 10 -ml specimen reservoir The disk was conditioned with 1 ml methanol and 1 ml of 0. 1 M phosphate buffer (p. H 6) A sample of 2 ml was applied to the cartridge Providing hydrophobic (C) and SCX interactions After dilution with 6 ml of the phosphate buffer

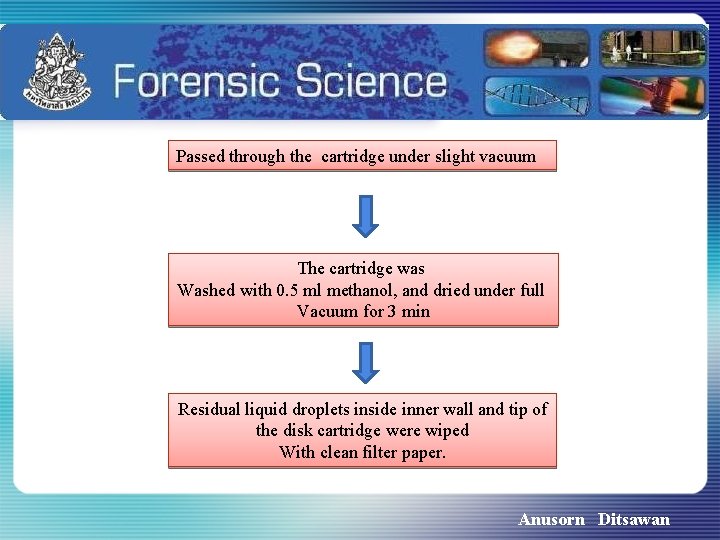
Passed through the cartridge under slight vacuum The cartridge was Washed with 0. 5 ml methanol, and dried under full Vacuum for 3 min Residual liquid droplets inside inner wall and tip of the disk cartridge were wiped With clean filter paper. Anusorn Ditsawan

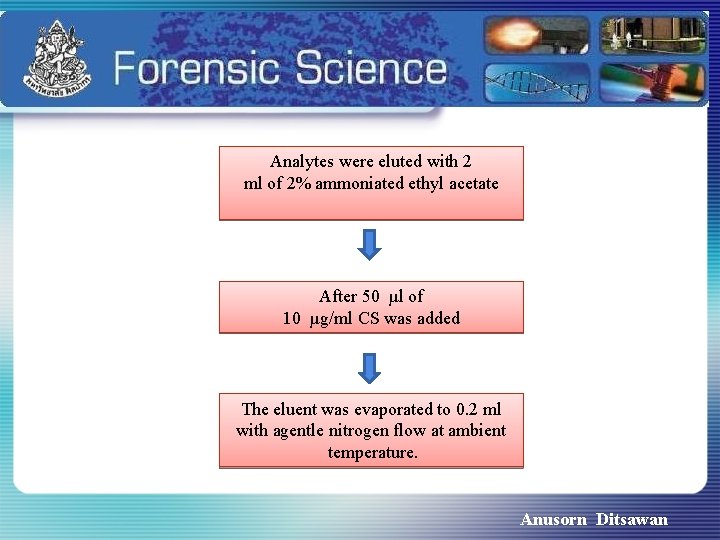
Analytes were eluted with 2 ml of 2% ammoniated ethyl acetate After 50 µl of 10 µg/ml CS was added The eluent was evaporated to 0. 2 ml with agentle nitrogen flow at ambient temperature. Anusorn Ditsawan

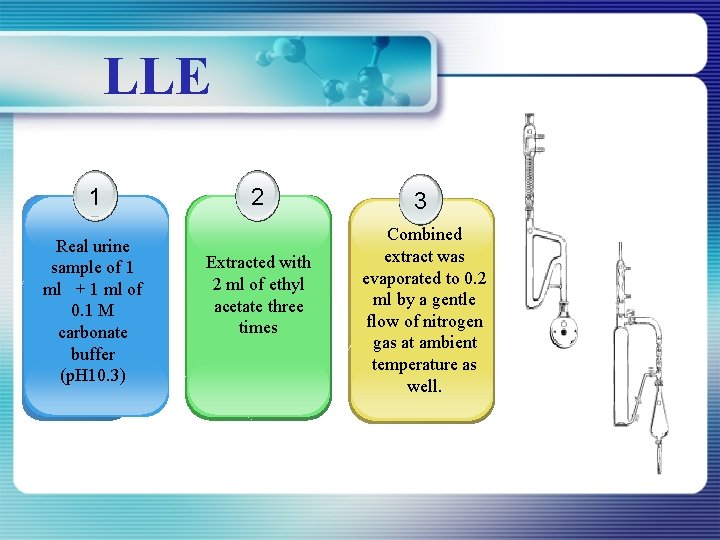
LLE 1 Real urine sample of 1 ml + 1 ml of 0. 1 M carbonate buffer (p. H 10. 3) 2 Extracted with 2 ml of ethyl acetate three times 3 Combined extract was evaporated to 0. 2 ml by a gentle flow of nitrogen gas at ambient temperature as well.

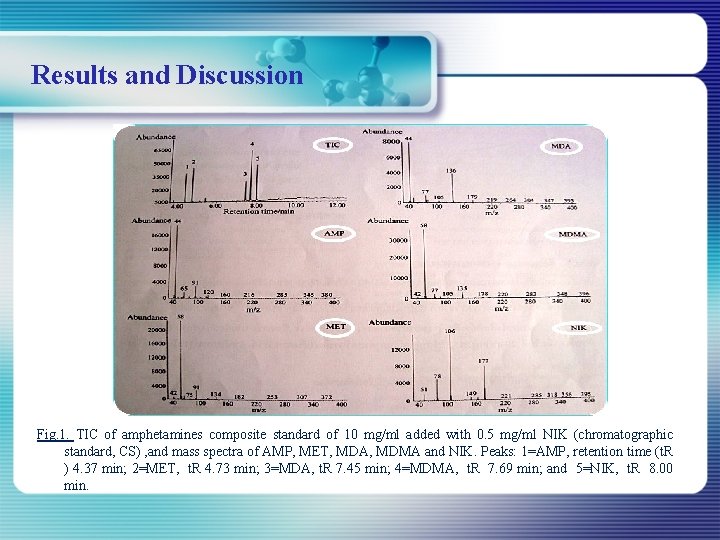
Results and Discussion Fig. 1. TIC of amphetamines composite standard of 10 mg/ml added with 0. 5 mg/ml NIK (chromatographic standard, CS) , and mass spectra of AMP, MET, MDA, MDMA and NIK. Peaks: 1=AMP, retention time (t. R ) 4. 37 min; 2=MET, t. R 4. 73 min; 3=MDA, t. R 7. 45 min; 4=MDMA, t. R 7. 69 min; and 5=NIK, t. R 8. 00 min.

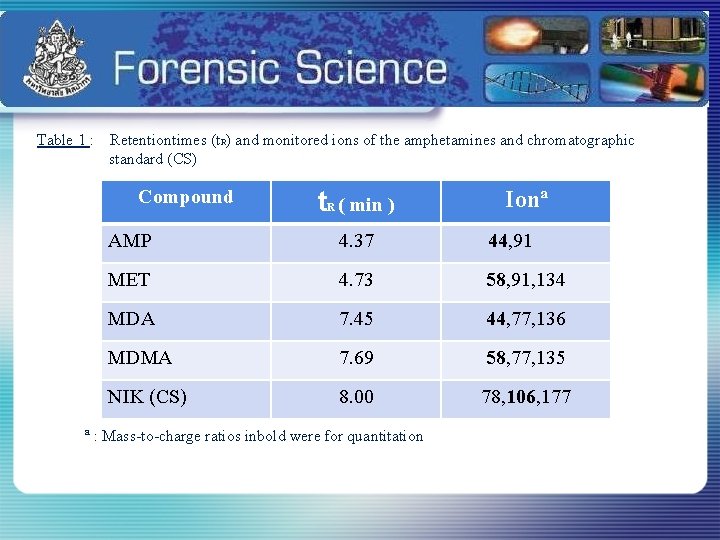
Table 1 : Retentiontimes (t. R) and monitored ions of the amphetamines and chromatographic standard (CS) Compound t R ( min ) Ionª AMP 4. 37 44, 91 MET 4. 73 58, 91, 134 MDA 7. 45 44, 77, 136 MDMA 7. 69 58, 77, 135 NIK (CS) 8. 00 78, 106, 177 ª : Mass-to-charge ratios inbold were for quantitation

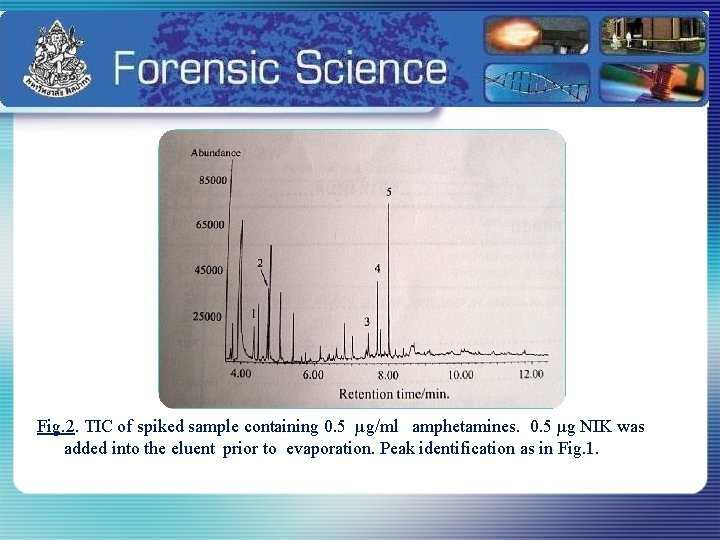
Fig. 2. TIC of spiked sample containing 0. 5 µg/ml amphetamines. 0. 5 µg NIK was added into the eluent prior to evaporation. Peak identification as in Fig. 1.

Ø The p. H and ion strength of the sample very important factors in SPE Ø A 6 -ml volume of 0. 1 M phosphate buffer (p. H 6) was added to 2 ml of sample to achieve proper p. H and ion strength Ø Inadequate dilution of the sample can cause low and nonreproducible recoveries (ex-periment observation)

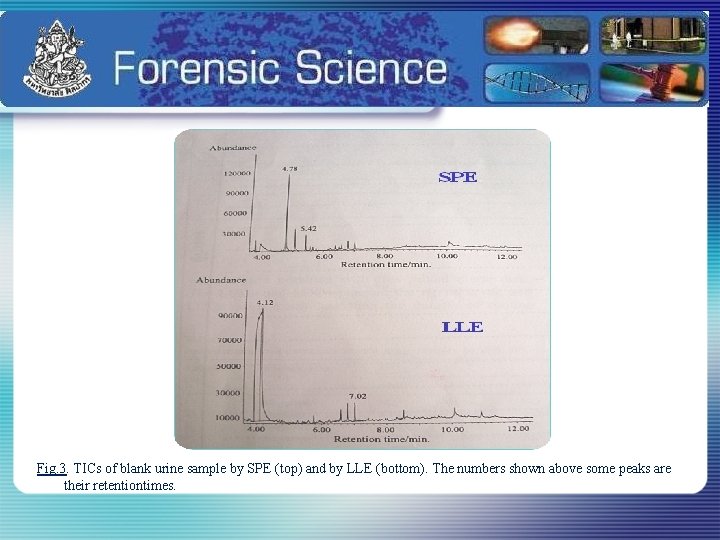
Fig. 3. TICs of blank urine sample by SPE (top) and by LLE (bottom). The numbers shown above some peaks are their retentiontimes.

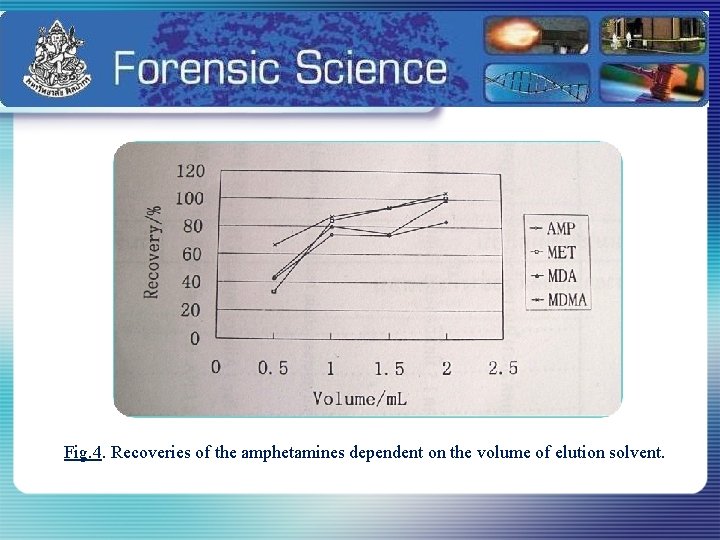
Fig. 4. Recoveries of the amphetamines dependent on the volume of elution solvent.

Compared with traditional SPE Ø Compared with traditional SPE , disk SPE required less solvents not only in conditioning and washing column but also in desorption of analytes (1– 2 ml vs 2– 6 ml) Ø The time needed to perform the SPE with an extraction disk is less than that needed with a classical SPE column from conditioning through desorption of extracts (8 min vs 20 min) Ø Care should be taken for disk SPE to prevent the column from running to dry before sample application otherwise air–water interfaces are formed which may affect recovery of analytes.

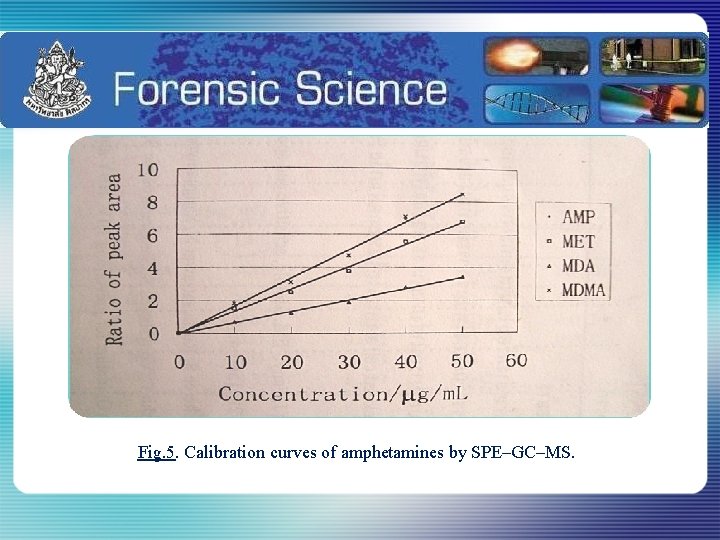
Fig. 5. Calibration curves of amphetamines by SPE–GC–MS.

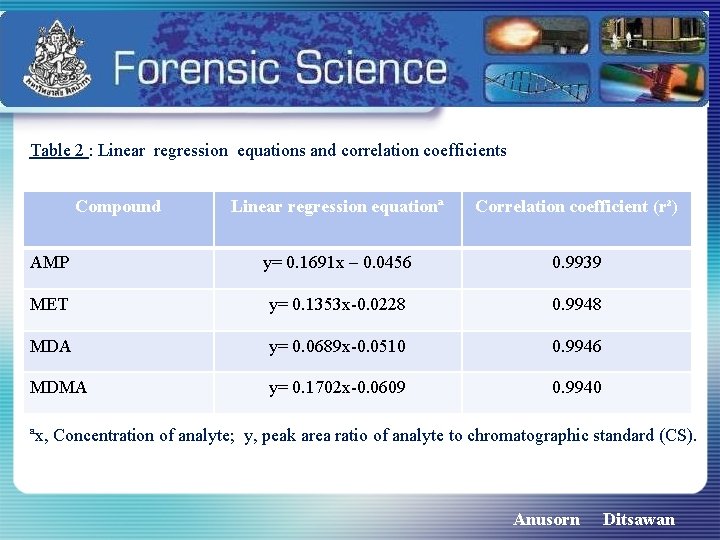
Table 2 : Linear regression equations and correlation coefficients Compound Linear regression equationª Correlation coefficient (r²) AMP y= 0. 1691 x – 0. 0456 0. 9939 MET y= 0. 1353 x-0. 0228 0. 9948 MDA y= 0. 0689 x-0. 0510 0. 9946 MDMA y= 0. 1702 x-0. 0609 0. 9940 ªx, Concentration of analyte; y, peak area ratio of analyte to chromatographic standard (CS). Anusorn Ditsawan

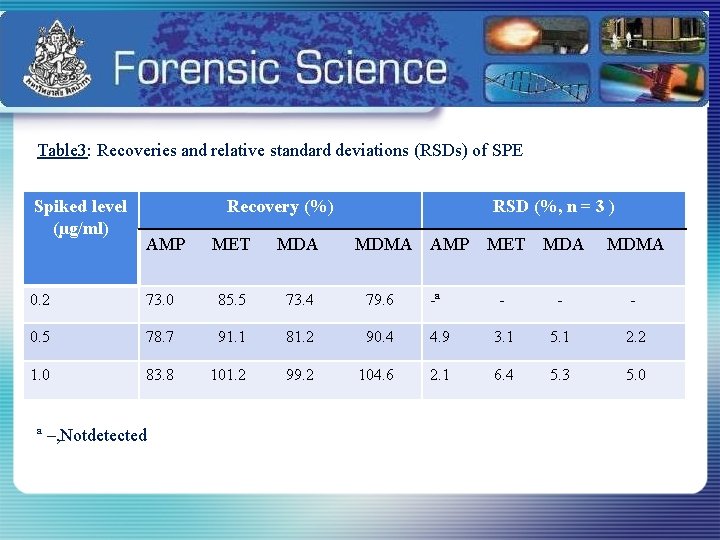
Table 3: Recoveries and relative standard deviations (RSDs) of SPE Spiked level (µg/ml) Recovery (%) AMP MET MDA RSD (%, n = 3 ) MDMA AMP MET MDA MDMA - - - 0. 2 73. 0 85. 5 73. 4 79. 6 -ª 0. 5 78. 7 91. 1 81. 2 90. 4 4. 9 3. 1 5. 1 2. 2 1. 0 83. 8 101. 2 99. 2 104. 6 2. 1 6. 4 5. 3 5. 0 ª –, Notdetected

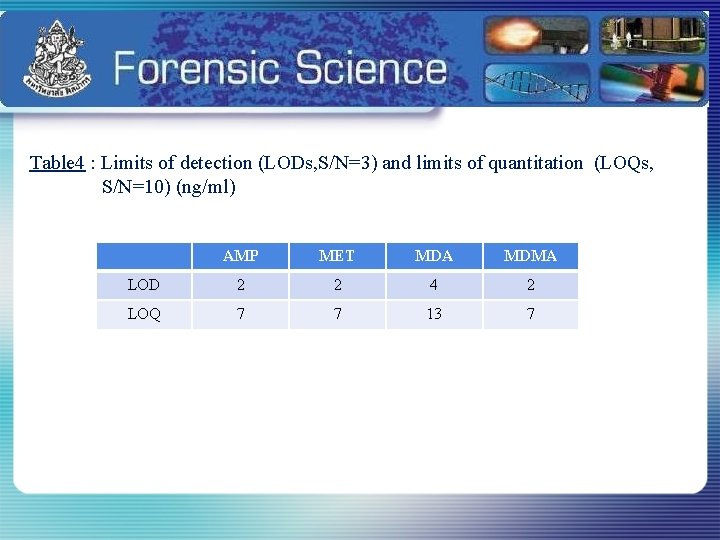
Table 4 : Limits of detection (LODs, S/N=3) and limits of quantitation (LOQs, S/N=10) (ng/ml) AMP MET MDA MDMA LOD 2 2 4 2 LOQ 7 7 13 7

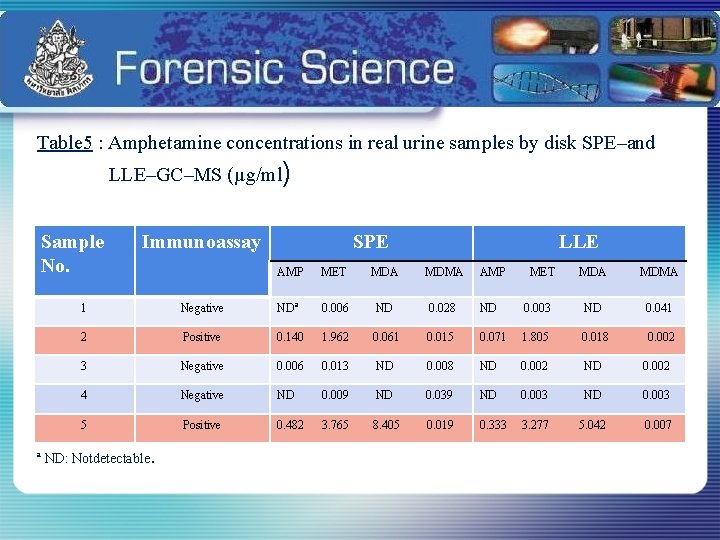
Table 5 : Amphetamine concentrations in real urine samples by disk SPE–and LLE–GC–MS (µg/ml) Sample No. Immunoassay SPE LLE AMP MET MDA MDMA 1 Negative NDª 0. 006 ND 0. 028 ND 0. 003 ND 0. 041 2 Positive 0. 140 1. 962 0. 061 0. 015 0. 071 1. 805 0. 018 0. 002 3 Negative 0. 006 0. 013 ND 0. 008 ND 0. 002 4 Negative ND 0. 009 ND 0. 039 ND 0. 003 5 Positive 0. 482 3. 765 8. 405 0. 019 0. 333 3. 277 5. 042 0. 007 ª ND: Notdetectable .

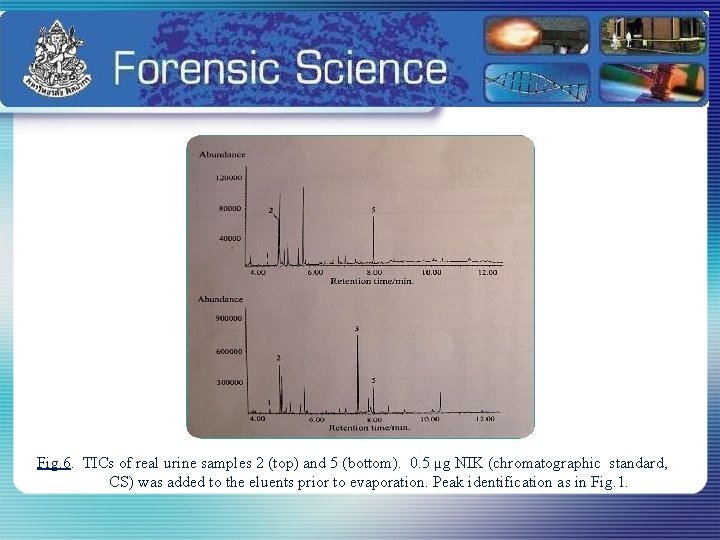
Fig. 6. TICs of real urine samples 2 (top) and 5 (bottom). 0. 5 µg NIK (chromatographic standard, CS) was added to the eluents prior to evaporation. Peak identification as in Fig. 1.

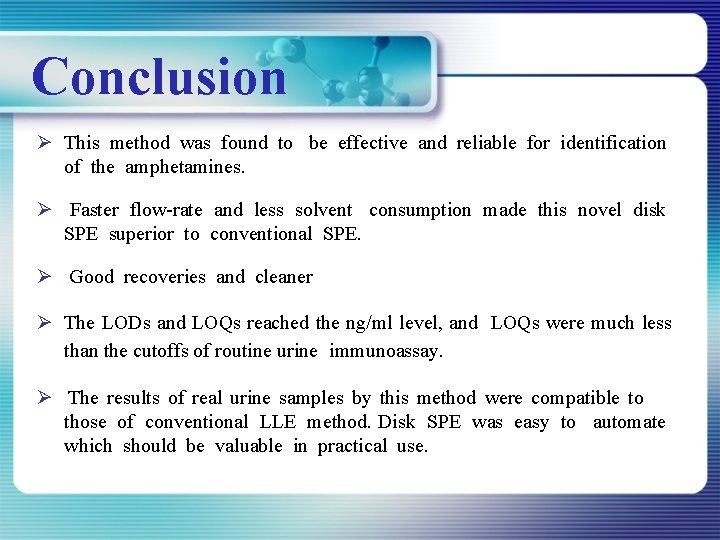
Conclusion Ø This method was found to be effective and reliable for identification of the amphetamines. Ø Faster flow-rate and less solvent consumption made this novel disk SPE superior to conventional SPE. Ø Good recoveries and cleaner Ø The LODs and LOQs reached the ng/ml level, and LOQs were much less than the cutoffs of routine urine immunoassay. Ø The results of real urine samples by this method were compatible to those of conventional LLE method. Disk SPE was easy to automate which should be valuable in practical use.

LOGO
 Visible signs of confirmation
Visible signs of confirmation Presentation of candidates for confirmation
Presentation of candidates for confirmation Outward sign of inward grace
Outward sign of inward grace Confirmation and fluency stage
Confirmation and fluency stage Surat confirmation letter
Surat confirmation letter Saint project for confirmation
Saint project for confirmation Staphylococcus aureus confirmation test
Staphylococcus aureus confirmation test Eucharist multiple choice questions
Eucharist multiple choice questions Confirmation blessing from parent
Confirmation blessing from parent Class opening prayer
Class opening prayer The sacrament of confirmation chapter 4
The sacrament of confirmation chapter 4 Baptism testimony examples
Baptism testimony examples Assert without proof
Assert without proof Closing confirmation letter
Closing confirmation letter Meeting jesus in the sacraments chapter 4
Meeting jesus in the sacraments chapter 4 Annointing of the sick symbols
Annointing of the sick symbols Verify death geeky medics
Verify death geeky medics Confirmation jeopardy
Confirmation jeopardy Exordium narratio confirmatio refutatio peroratio
Exordium narratio confirmatio refutatio peroratio Confirmation sacrament symbols
Confirmation sacrament symbols Levels of confirmation
Levels of confirmation Introduction narration confirmation refutation conclusion
Introduction narration confirmation refutation conclusion Staffan göjeryd
Staffan göjeryd Confirmation bias
Confirmation bias Third party verification letter sample
Third party verification letter sample Confirmation sacrament
Confirmation sacrament Confirmation of estrangement form 2021/22
Confirmation of estrangement form 2021/22 On-site confirmation of flow diagram
On-site confirmation of flow diagram Verify udin icai
Verify udin icai Purchase order collaboration
Purchase order collaboration Confirmation test
Confirmation test Ethiopian police university college website
Ethiopian police university college website Faculty of veterinary medicine cairo university logo
Faculty of veterinary medicine cairo university logo Amirkabir university of technology logo
Amirkabir university of technology logo Amirkabir university of technology logo
Amirkabir university of technology logo University logo
University logo Digital signal processing
Digital signal processing