Lesson 3 Title Calculating Rate of Reaction LI

- Slides: 15

Lesson 3 Title: Calculating Rate of Reaction LI: Calculate average rate of reaction Green – I can describe how the rate of a reaction can be followed and measured. I can interpret graphs of reactions. I can carry calculate the average rate of a reaction. Amber - I can describe how the rate of a reaction can be followed and measured. I can interpret graphs of reactions. Red - I can describe how the rate of a reaction can be followed and measured.

Following the Progress of a Reaction using Graphs • Rate of the reaction – The steeper the slope the faster the reaction • Completion of the reaction – When the graph levels off the reaction has stopped – Usually one or both of the reactants has been used up

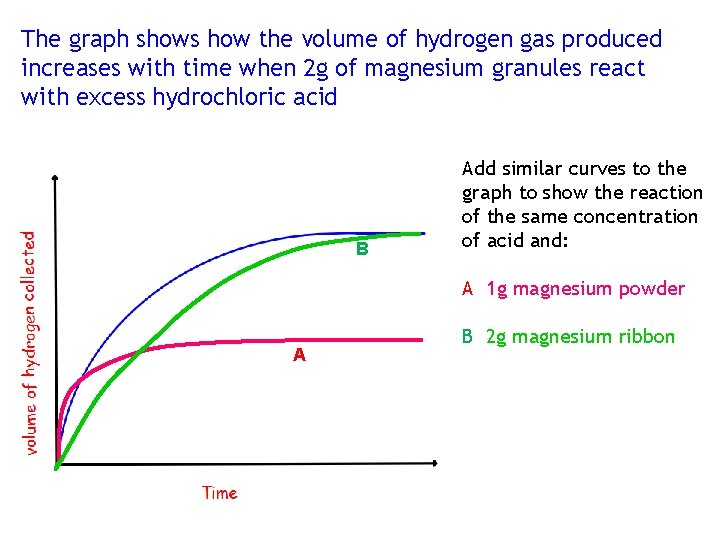
The graph shows how the volume of hydrogen gas produced increases with time when 2 g of magnesium granules react with excess hydrochloric acid B Add similar curves to the graph to show the reaction of the same concentration of acid and: A 1 g magnesium powder A B 2 g magnesium ribbon

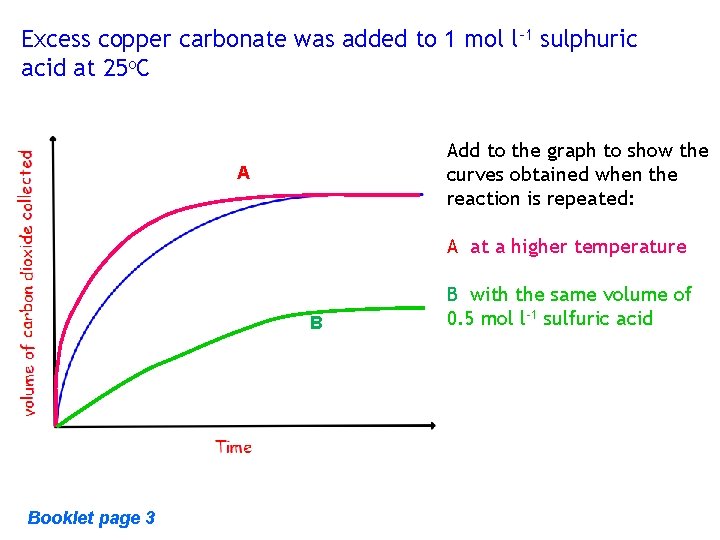
Excess copper carbonate was added to 1 mol l-1 sulphuric acid at 25 o. C Add to the graph to show the curves obtained when the reaction is repeated: A A at a higher temperature B Booklet page 3 B with the same volume of 0. 5 mol l-1 sulfuric acid

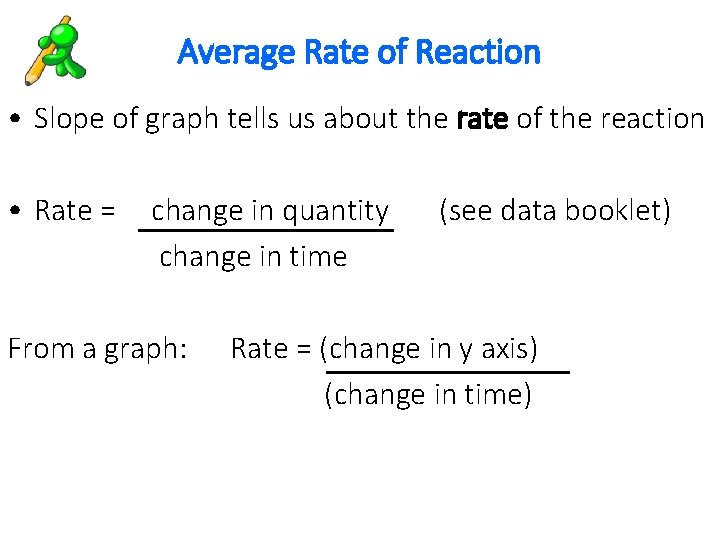
Average Rate of Reaction • Slope of graph tells us about the rate of the reaction • Rate = change in quantity change in time From a graph: (see data booklet) Rate = (change in y axis) (change in time)

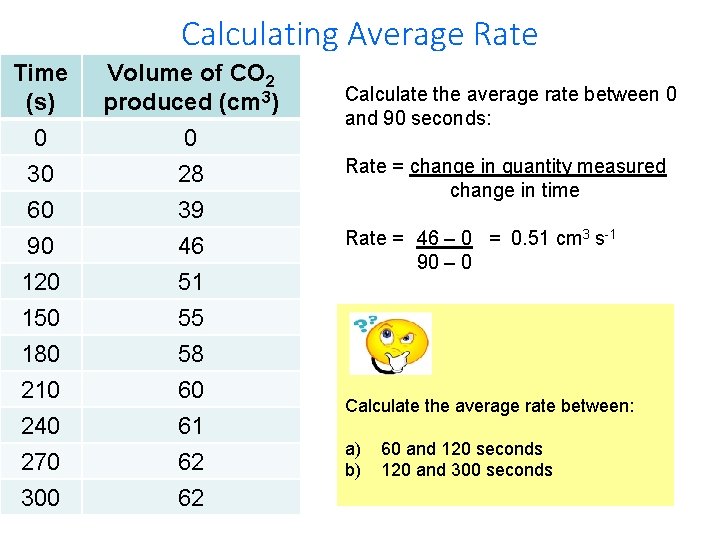
Calculating Average Rate Time (s) 0 Volume of CO 2 produced (cm 3) 0 30 60 90 120 150 180 210 240 270 300 28 39 46 51 55 58 60 61 62 62 Calculate the average rate between 0 and 90 seconds: Rate = change in quantity measured change in time Rate = 46 – 0 = 0. 51 cm 3 s-1 90 – 0 Calculate the average rate between: a) b) 60 and 120 seconds 120 and 300 seconds

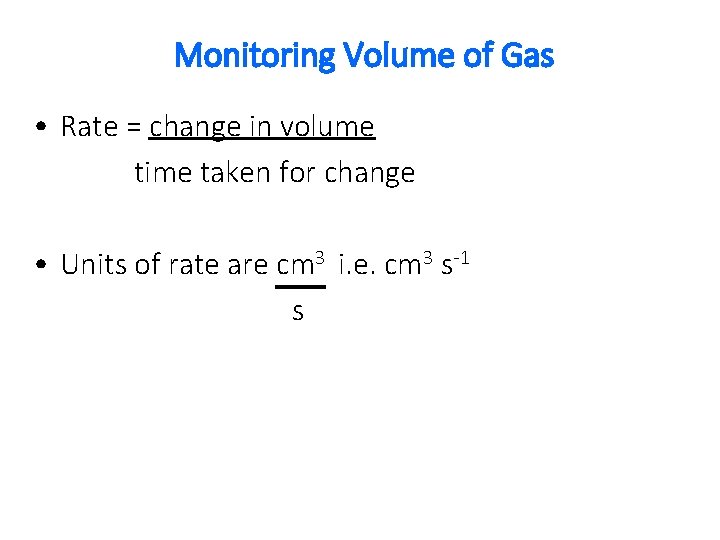
Monitoring Volume of Gas • Rate = change in volume time taken for change • Units of rate are cm 3 i. e. cm 3 s-1 s

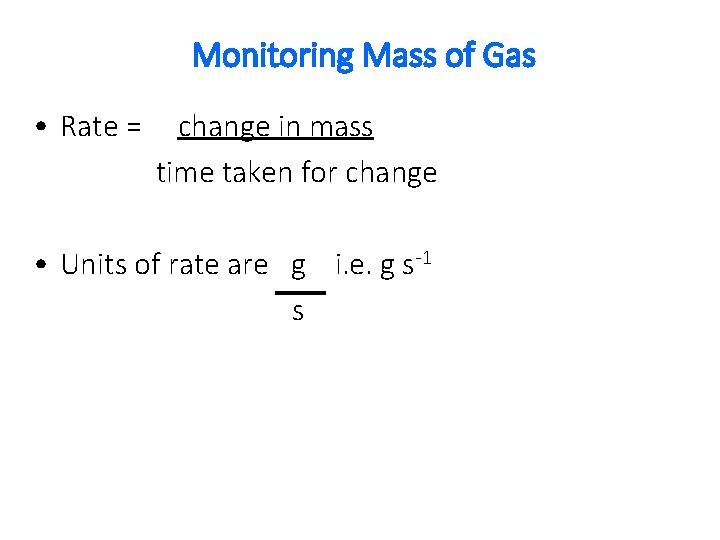
Monitoring Mass of Gas • Rate = change in mass time taken for change • Units of rate are g i. e. g s-1 s

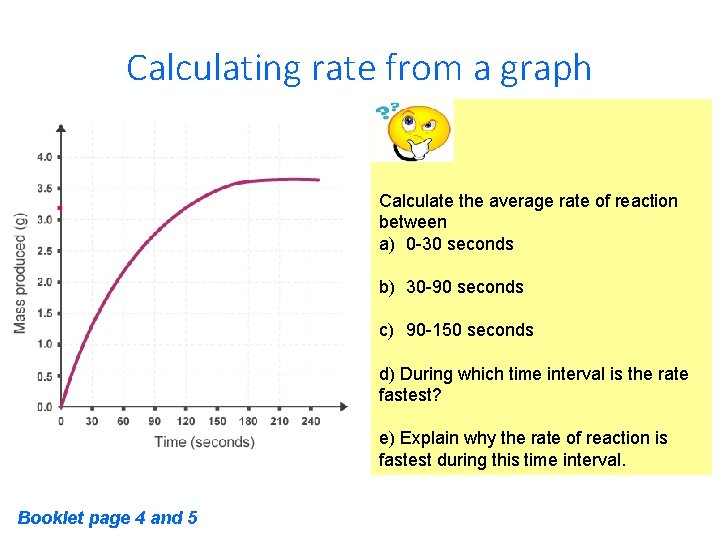
Calculating rate from a graph Calculate the average rate of reaction between a) 0 -30 seconds b) 30 -90 seconds c) 90 -150 seconds d) During which time interval is the rate fastest? e) Explain why the rate of reaction is fastest during this time interval. Booklet page 4 and 5

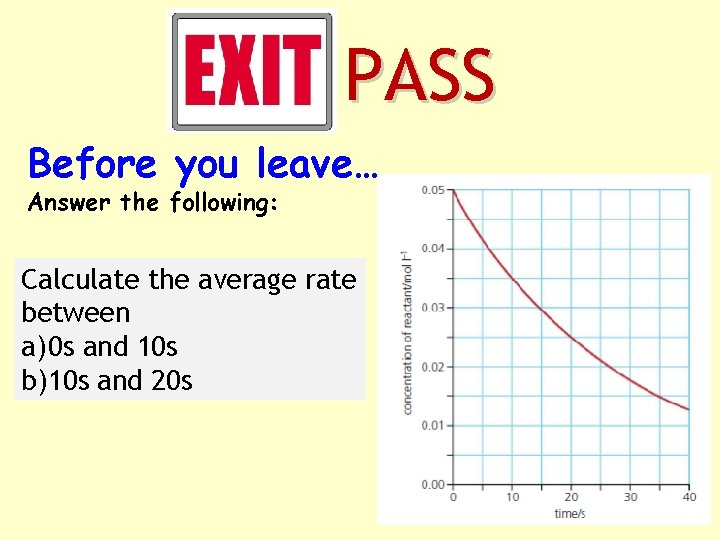
PASS Before you leave… Answer the following: Calculate the average rate between a) 0 s and 10 s b)10 s and 20 s

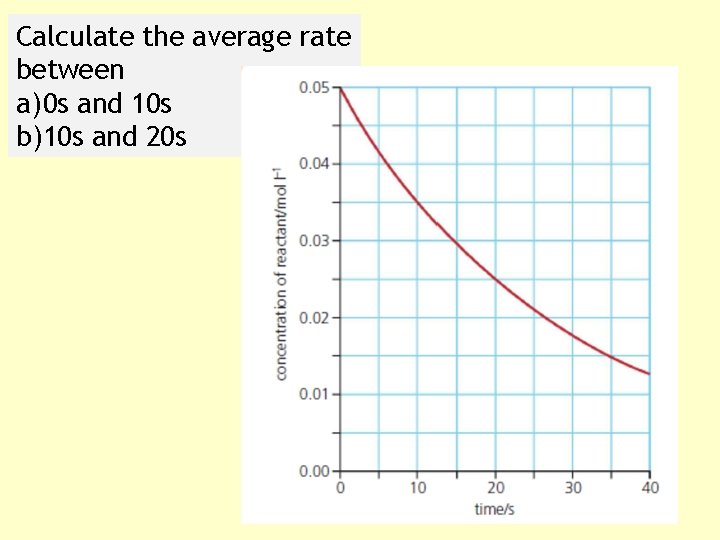
Calculate the average rate between a) 0 s and 10 s b)10 s and 20 s


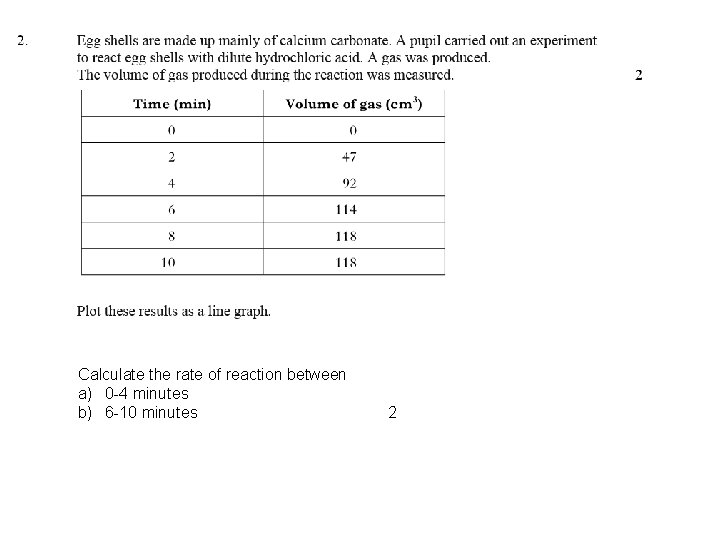
Calculate the rate of reaction between a) 0 -4 minutes b) 6 -10 minutes 2


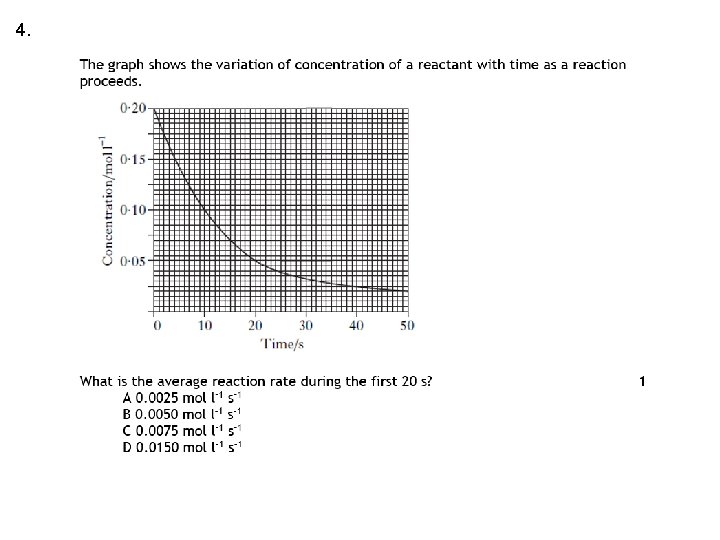
4.
 Order of reaction
Order of reaction How to find the enthalpy change of a reaction
How to find the enthalpy change of a reaction 17.4 calculating heats of reaction
17.4 calculating heats of reaction Calculate inflation rate
Calculate inflation rate How to calculate iv push rate
How to calculate iv push rate Drip rate formula
Drip rate formula Infusion rate formula
Infusion rate formula How to calculate absorption cost per unit
How to calculate absorption cost per unit Calculating iv infusion rate
Calculating iv infusion rate Gtt/min formula
Gtt/min formula Rate of infusion
Rate of infusion How to find predetermined overhead rate
How to find predetermined overhead rate Machine-hour
Machine-hour Calculating average rate of change
Calculating average rate of change Prefatory and supplementary part of proposal
Prefatory and supplementary part of proposal Title title
Title title