Length Scale of Imperfections Line Defects Dislocations and
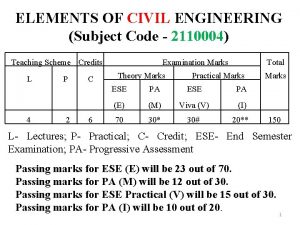
- Slides: 25

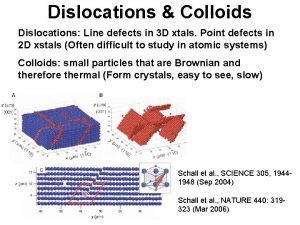
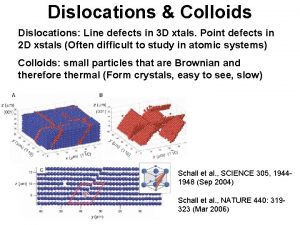
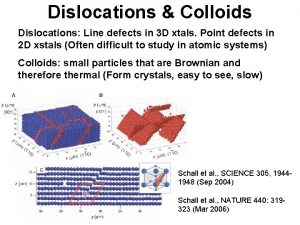
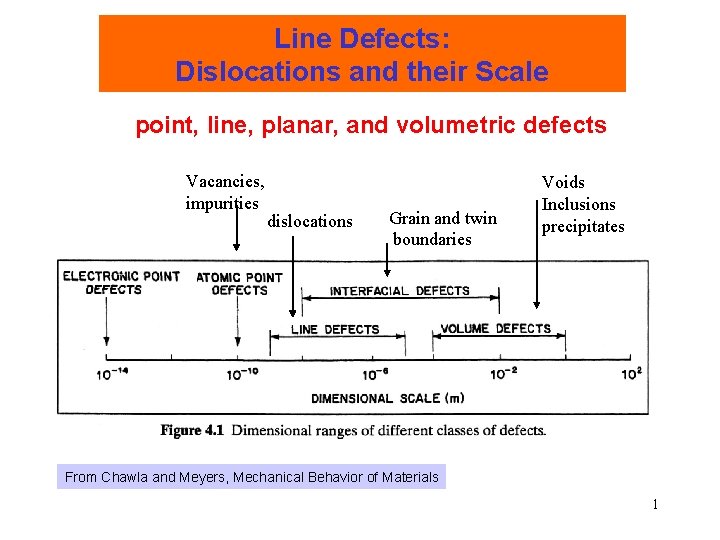
Length Scale of Imperfections Line Defects: Dislocations and their Scale point, line, planar, and volumetric defects Vacancies, impurities dislocations Grain and twin boundaries Voids Inclusions precipitates From Chawla and Meyers, Mechanical Behavior of Materials 1

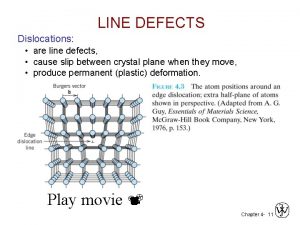
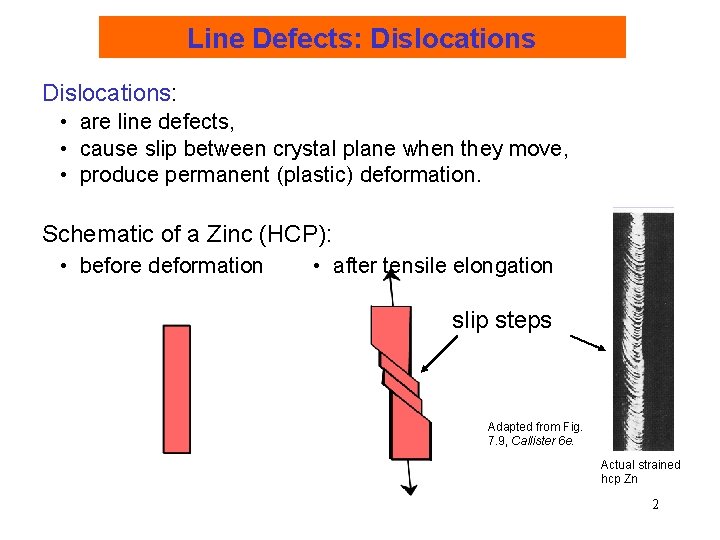
Line Defects: Dislocations: • are line defects, • cause slip between crystal plane when they move, • produce permanent (plastic) deformation. Schematic of a Zinc (HCP): • before deformation • after tensile elongation slip steps Adapted from Fig. 7. 9, Callister 6 e. Actual strained hcp Zn 2

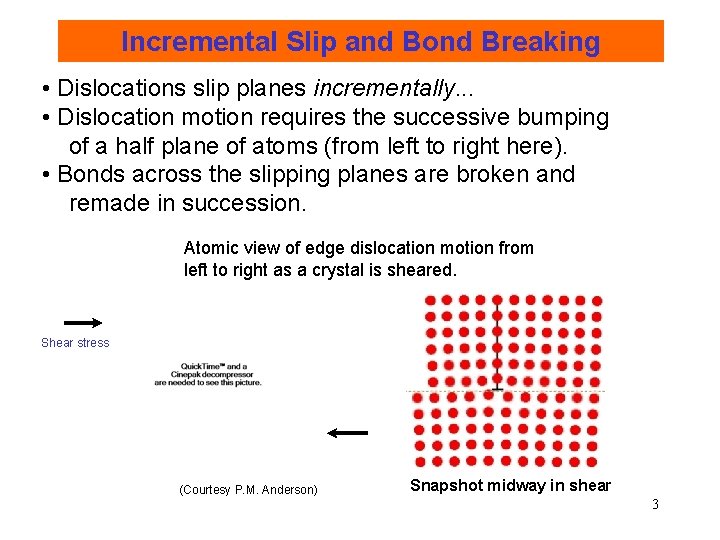
Incremental Slip and Bond Breaking • Dislocations slip planes incrementally. . . • Dislocation motion requires the successive bumping of a half plane of atoms (from left to right here). • Bonds across the slipping planes are broken and remade in succession. Atomic view of edge dislocation motion from left to right as a crystal is sheared. Shear stress (Courtesy P. M. Anderson) Snapshot midway in shear 3

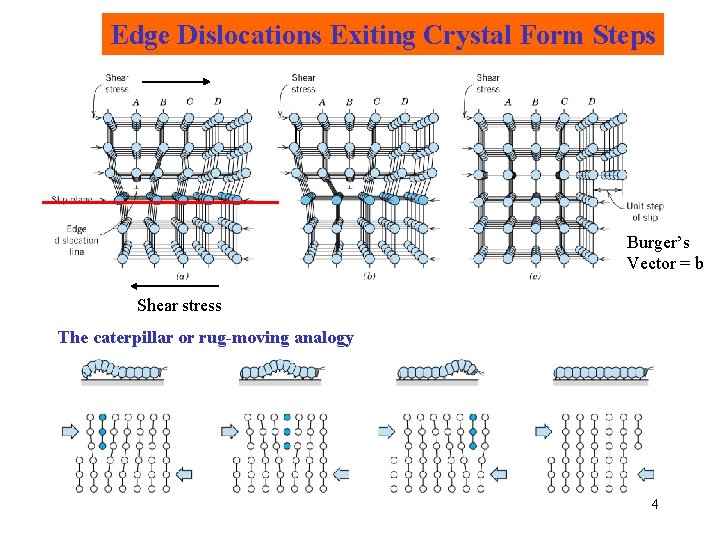
Edge Dislocations Exiting Crystal Form Steps Burger’s Vector = b Shear stress The caterpillar or rug-moving analogy 4

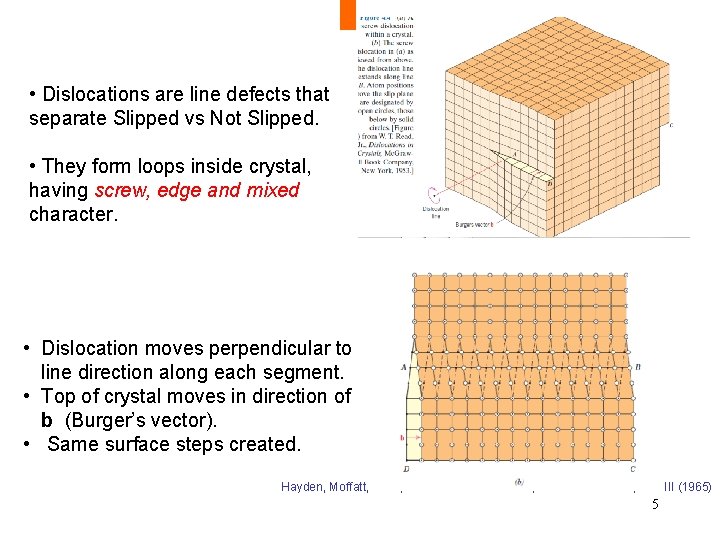
• Dislocations are line defects that separate Slipped vs Not Slipped. • They form loops inside crystal, having screw, edge and mixed character. • Dislocation moves perpendicular to line direction along each segment. • Top of crystal moves in direction of b (Burger’s vector). • Same surface steps created. Hayden, Moffatt, Wulff, “The Structure and Properties of Materials, ” Vol III (1965) 5

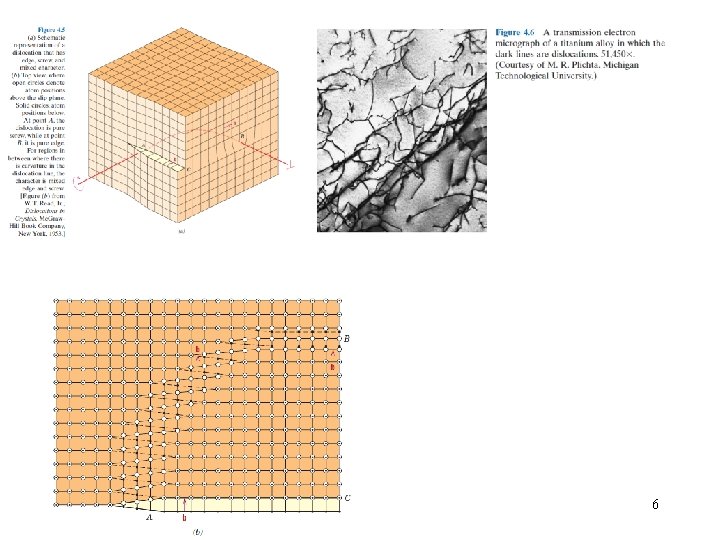
6

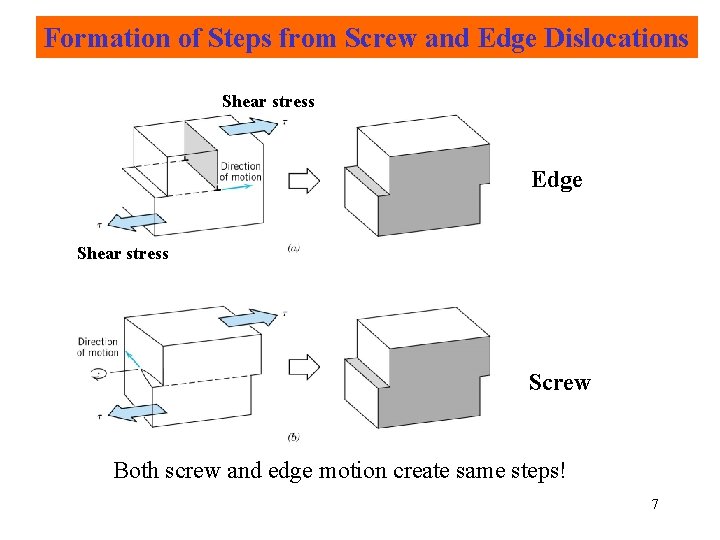
Formation of Steps from Screw and Edge Dislocations Shear stress Edge Shear stress Screw Both screw and edge motion create same steps! 7

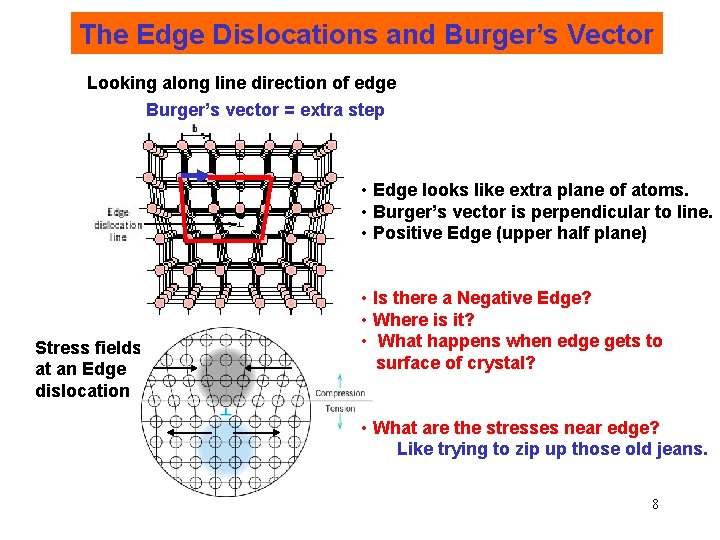
The Edge Dislocations and Burger’s Vector Looking along line direction of edge Burger’s vector = extra step • Edge looks like extra plane of atoms. • Burger’s vector is perpendicular to line. • Positive Edge (upper half plane) Stress fields at an Edge dislocation • Is there a Negative Edge? • Where is it? • What happens when edge gets to surface of crystal? • What are the stresses near edge? Like trying to zip up those old jeans. 8

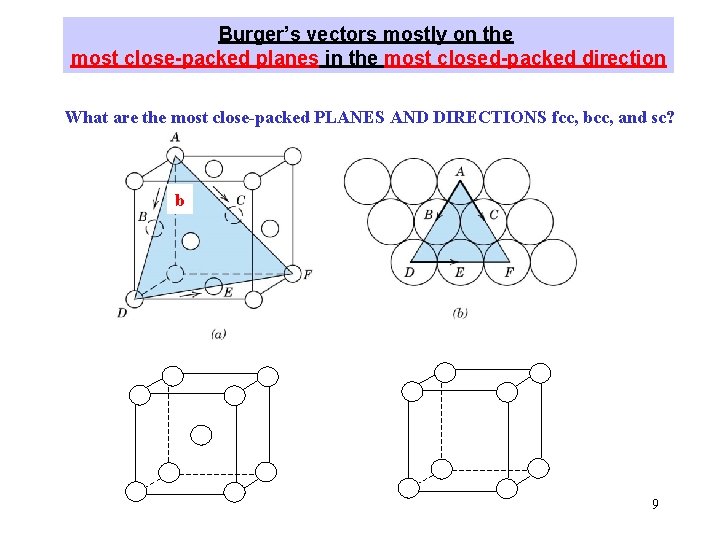
Burger’s vectors mostly on the most close-packed planes in the most closed-packed direction What are the most close-packed PLANES AND DIRECTIONS fcc, bcc, and sc? b 9

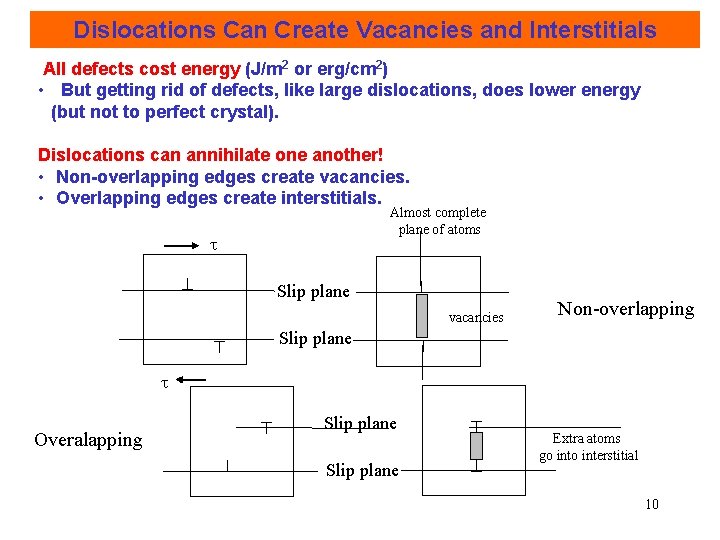
Dislocations Can Create Vacancies and Interstitials All defects cost energy (J/m 2 or erg/cm 2) • But getting rid of defects, like large dislocations, does lower energy (but not to perfect crystal). Dislocations can annihilate one another! • Non-overlapping edges create vacancies. • Overlapping edges create interstitials. Almost complete plane of atoms Slip plane vacancies Slip plane Non-overlapping Overalapping Slip plane Extra atoms go interstitial 10

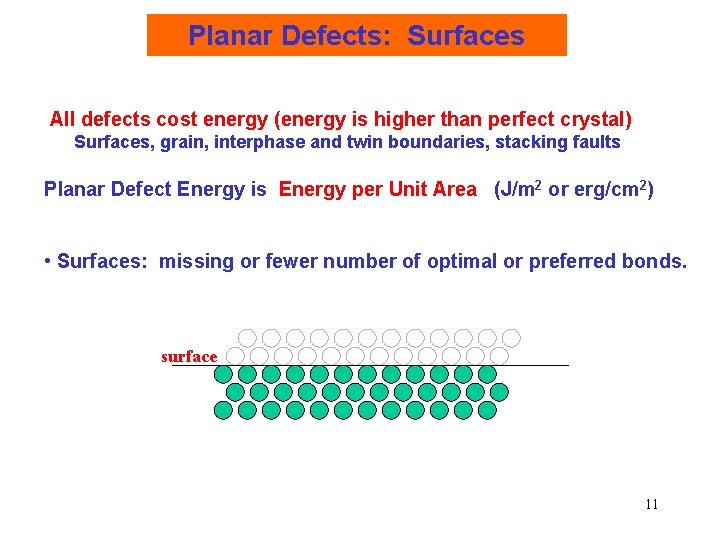
Planar Defects: Surfaces All defects cost energy (energy is higher than perfect crystal) Surfaces, grain, interphase and twin boundaries, stacking faults Planar Defect Energy is Energy per Unit Area (J/m 2 or erg/cm 2) • Surfaces: missing or fewer number of optimal or preferred bonds. surface 11

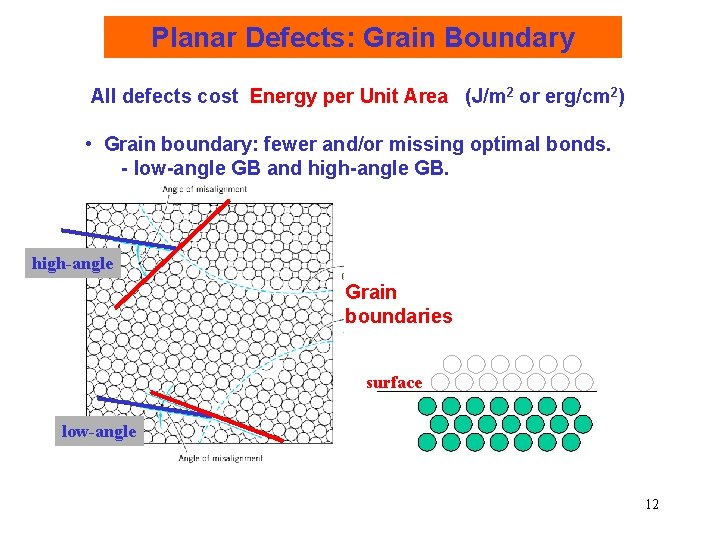
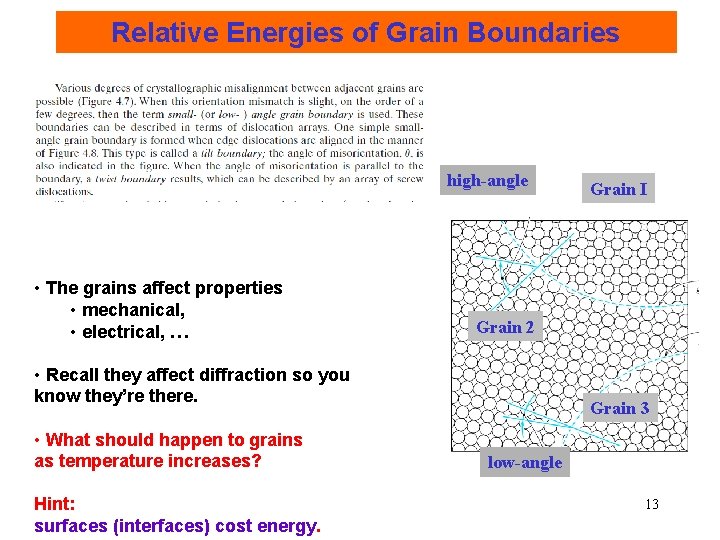
Planar Defects: Grain Boundary All defects cost Energy per Unit Area (J/m 2 or erg/cm 2) • Grain boundary: fewer and/or missing optimal bonds. - low-angle GB and high-angle GB. high-angle Grain boundaries surface low-angle 12

Relative Energies of Grain Boundaries high-angle • The grains affect properties • mechanical, • electrical, … Grain 2 • Recall they affect diffraction so you know they’re there. • What should happen to grains as temperature increases? Hint: surfaces (interfaces) cost energy. Grain I Grain 3 low-angle 13

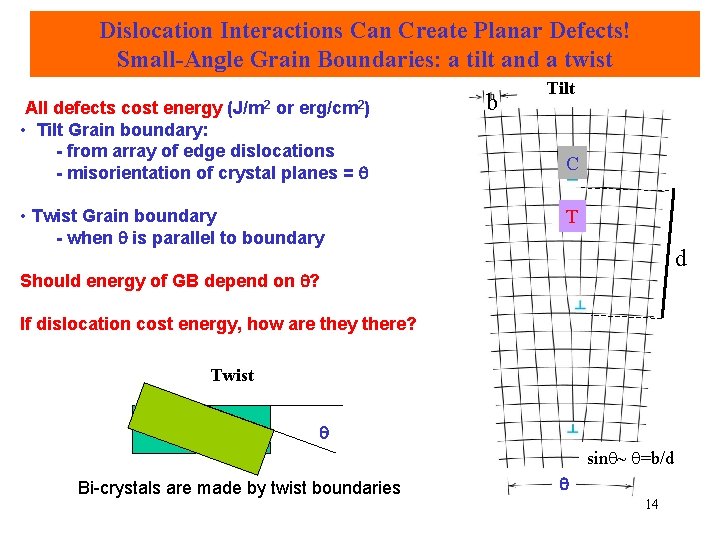
Dislocation Interactions Can Create Planar Defects! Small-Angle Grain Boundaries: a tilt and a twist All defects cost energy (J/m 2 or erg/cm 2) • Tilt Grain boundary: - from array of edge dislocations - misorientation of crystal planes = • Twist Grain boundary - when is parallel to boundary b Tilt C T d Should energy of GB depend on ? If dislocation cost energy, how are they there? Twist sin ~ =b/d Bi-crystals are made by twist boundaries 14

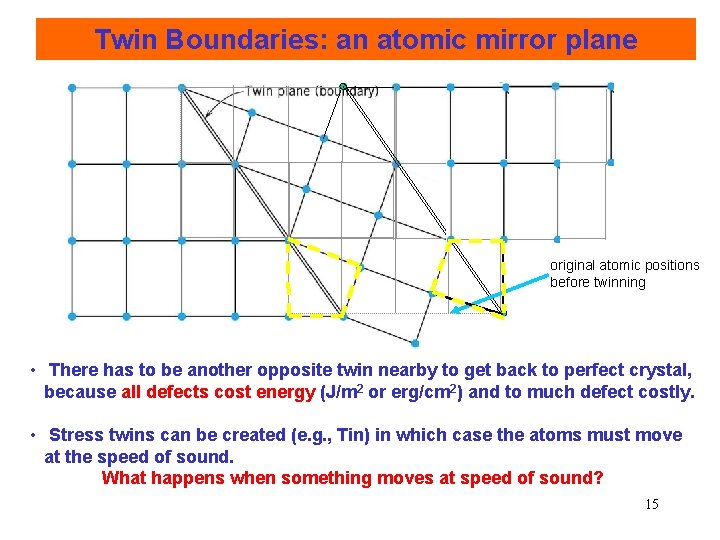
Twin Boundaries: an atomic mirror plane original atomic positions before twinning • There has to be another opposite twin nearby to get back to perfect crystal, because all defects cost energy (J/m 2 or erg/cm 2) and to much defect costly. • Stress twins can be created (e. g. , Tin) in which case the atoms must move at the speed of sound. What happens when something moves at speed of sound? 15

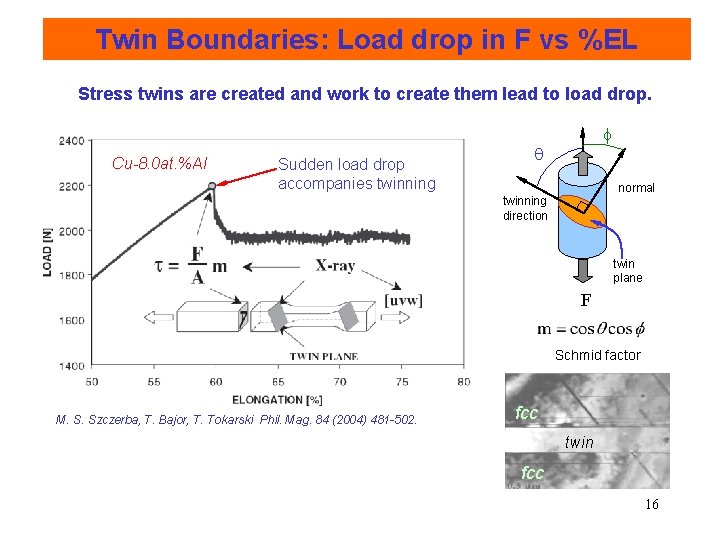
Twin Boundaries: Load drop in F vs %EL Stress twins are created and work to create them lead to load drop. Cu-8. 0 at. %Al Sudden load drop accompanies twinning f normal twinning direction twin plane F Schmid factor M. S. Szczerba, T. Bajor, T. Tokarski Phil. Mag. 84 (2004) 481 -502. fcc twin fcc 16

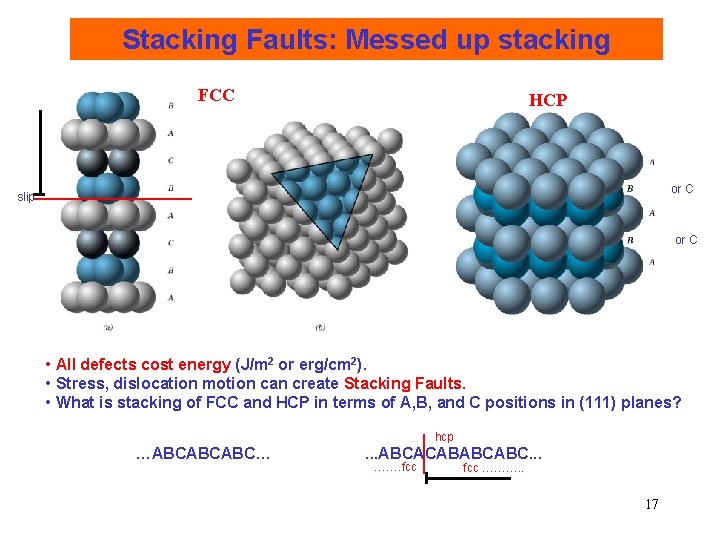
Stacking Faults: Messed up stacking FCC HCP or C slip or C • All defects cost energy (J/m 2 or erg/cm 2). • Stress, dislocation motion can create Stacking Faults. • What is stacking of FCC and HCP in terms of A, B, and C positions in (111) planes? hcp …ABCABCABC… . . . ABCACABABCABC. . . ……. fcc ………. . 17

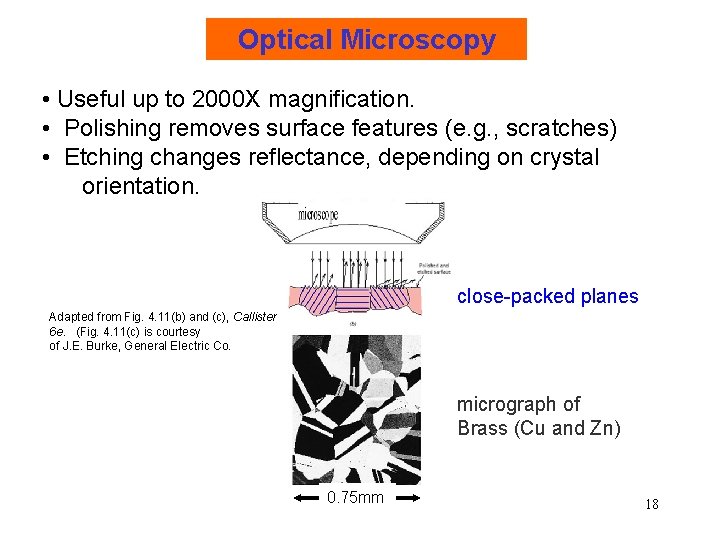
Optical Microscopy • Useful up to 2000 X magnification. • Polishing removes surface features (e. g. , scratches) • Etching changes reflectance, depending on crystal orientation. close-packed planes Adapted from Fig. 4. 11(b) and (c), Callister 6 e. (Fig. 4. 11(c) is courtesy of J. E. Burke, General Electric Co. micrograph of Brass (Cu and Zn) 0. 75 mm 18

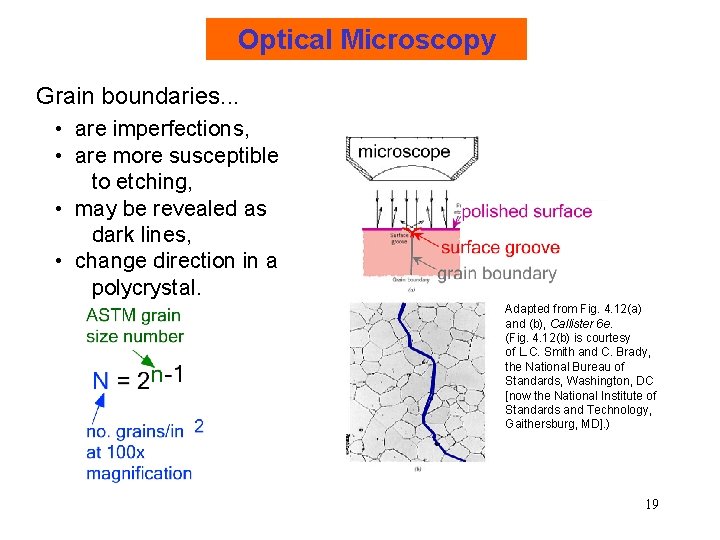
Optical Microscopy Grain boundaries. . . • are imperfections, • are more susceptible to etching, • may be revealed as dark lines, • change direction in a polycrystal. Adapted from Fig. 4. 12(a) and (b), Callister 6 e. (Fig. 4. 12(b) is courtesy of L. C. Smith and C. Brady, the National Bureau of Standards, Washington, DC [now the National Institute of Standards and Technology, Gaithersburg, MD]. ) 19

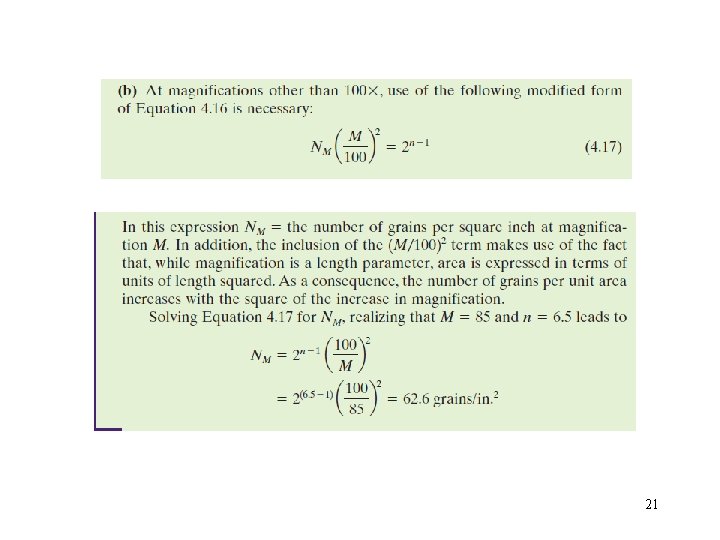
20

21

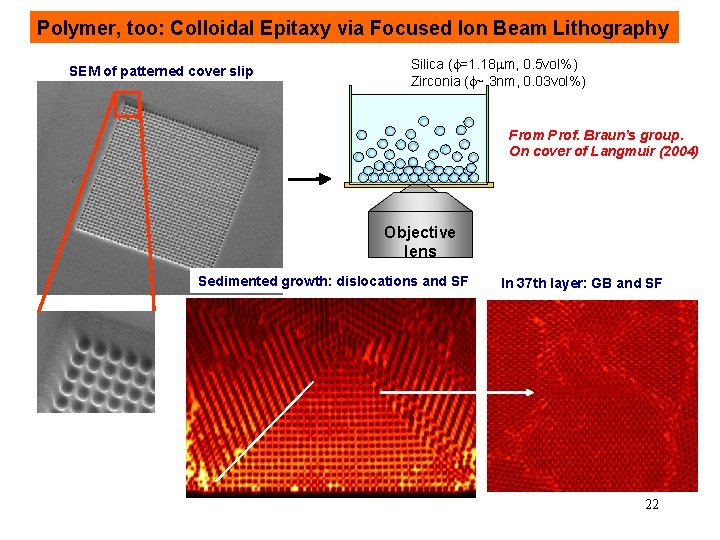
Polymer, too: Colloidal Epitaxy via Focused Ion Beam Lithography SEM of patterned cover slip Silica (f=1. 18 mm, 0. 5 vol%) Zirconia (f~ 3 nm, 0. 03 vol%) From Prof. Braun’s group. On cover of Langmuir (2004) Objective lens Sedimented growth: dislocations and SF In 37 th layer: GB and SF 22

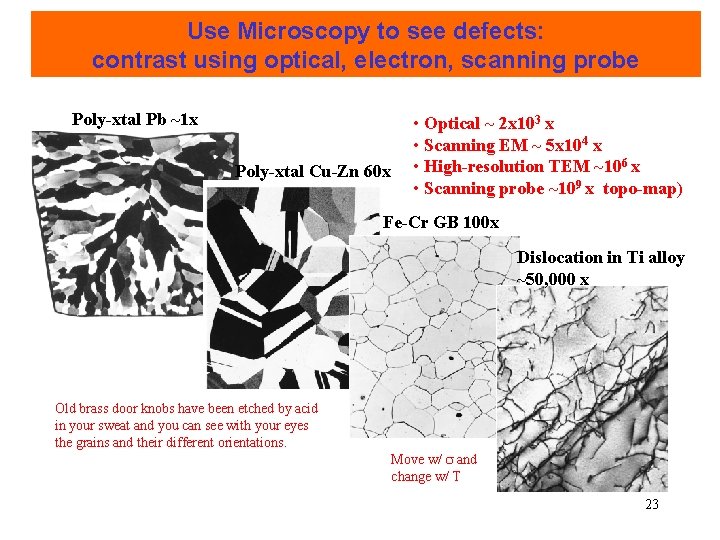
Use Microscopy to see defects: contrast using optical, electron, scanning probe Poly-xtal Pb ~1 x Poly-xtal Cu-Zn 60 x • Optical ~ 2 x 103 x • Scanning EM ~ 5 x 104 x • High-resolution TEM ~106 x • Scanning probe ~109 x topo-map) Fe-Cr GB 100 x Dislocation in Ti alloy ~50, 000 x Old brass door knobs have been etched by acid in your sweat and you can see with your eyes the grains and their different orientations. Move w/ and change w/ T 23

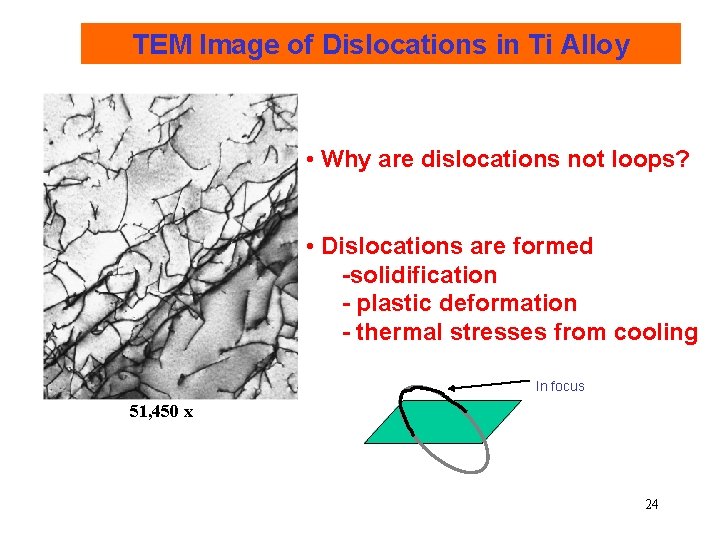
TEM Image of Dislocations in Ti Alloy • Why are dislocations not loops? • Dislocations are formed -solidification - plastic deformation - thermal stresses from cooling In focus 51, 450 x 24

SUMMARY • Defect materials responsible for most desired properties useful to engineering, e. g. , mechanical, thermal, and electrical. • They occur in metals, ceramics, polymers, and semiconductors. • Defect can be categorized in terms of Point, Line, or Planar defects. • Point: vacancies, interstitials, substitutional, … • Line: dislocations (mostly for metals, but not exclusively). • Planar: surfaces, grain boundaries, boundaries (twin, antiphase, domain, tilt … ), stacking faults, … • Defects can be observed by eye or various microscopies. • Defects can be created or affected by temperature, stress, etc. , requiring or leading to other defects, as with dislocations. 25
 Dislocations in crystals
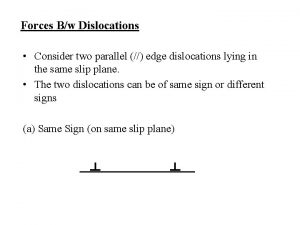
Dislocations in crystals Consider two edge dislocations of opposite sign
Consider two edge dislocations of opposite sign Wendelin wright
Wendelin wright Types of imperfection
Types of imperfection Volume imperfection
Volume imperfection Supplementary design
Supplementary design Lattice imperfections
Lattice imperfections What is the ratio of the length of to the length of ?
What is the ratio of the length of to the length of ? A plain scale of 1cm=5m and show on it 37m
A plain scale of 1cm=5m and show on it 37m Single electron transistor
Single electron transistor Scale up and scale out in hadoop
Scale up and scale out in hadoop Plain scale can measure upto
Plain scale can measure upto Datascale systems
Datascale systems Proper protractor orientation
Proper protractor orientation Understanding scale drawings
Understanding scale drawings Product consistency example
Product consistency example Line inclined to both hp and vp
Line inclined to both hp and vp A line pq 75mm long
A line pq 75mm long Product service mix
Product service mix Cut to length line definition
Cut to length line definition Projection of lines inclined to both the planes
Projection of lines inclined to both the planes Schottky and frenkel defects
Schottky and frenkel defects Unacceptable weld profile
Unacceptable weld profile Cuts and washes defects in casting
Cuts and washes defects in casting Differences between schottky defect and frenkel defect
Differences between schottky defect and frenkel defect Shell cleanliness shell soundness shell texture shell shape
Shell cleanliness shell soundness shell texture shell shape