Kepler 1 planet with two suns Homework 3




















- Slides: 31

Kepler 1: planet with two suns

Homework #3 Due Wednesday, 11: 00 p. m. Answers to all homework questions will be posted on the class website First exam: Monday, October 3.

Every element has multiple isotopes v same number of protons (same element) v different numbers of neutrons

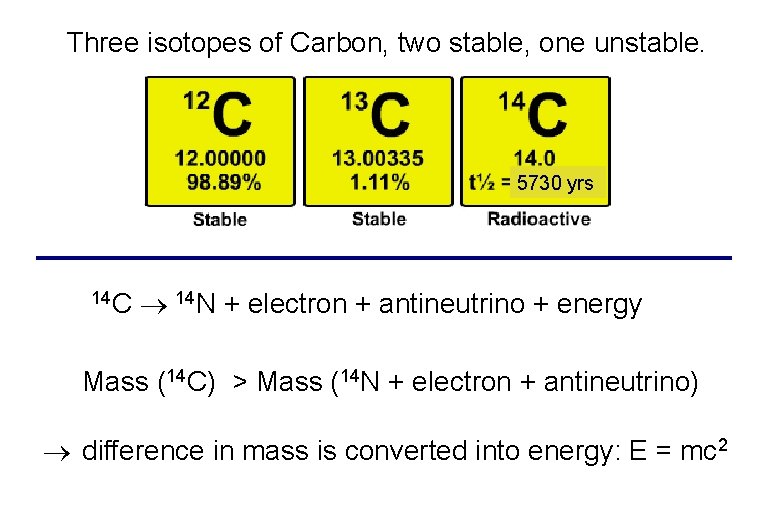
Three isotopes of Carbon, two stable, one unstable. 5730 yrs 14 C 14 N + electron + antineutrino + energy Mass (14 C) > Mass (14 N + electron + antineutrino) difference in mass is converted into energy: E = mc 2

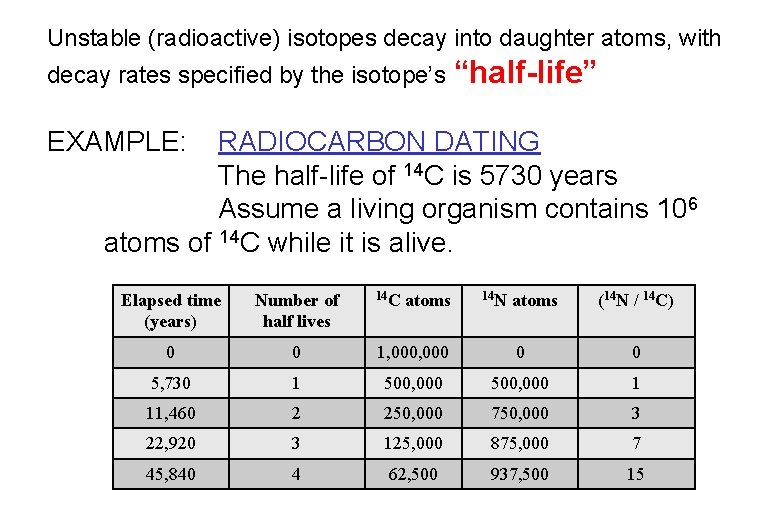
Unstable (radioactive) isotopes decay into daughter atoms, with decay rates specified by the isotope’s “half-life” EXAMPLE: RADIOCARBON DATING The half-life of 14 C is 5730 years Assume a living organism contains 106 atoms of 14 C while it is alive. Elapsed time (years) Number of half lives 14 C atoms 14 N atoms (14 N / 14 C) 0 0 1, 000 0 0 5, 730 1 500, 000 1 11, 460 2 250, 000 750, 000 3 22, 920 3 125, 000 875, 000 7 45, 840 4 62, 500 937, 500 15

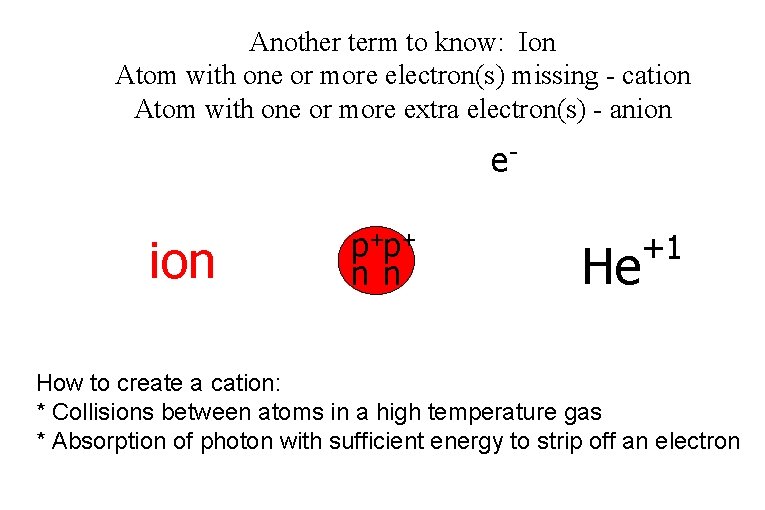
Another term to know: Ion Atom with one or more electron(s) missing - cation Atom with one or more extra electron(s) - anion e- ion p+p+ n n He +1 How to create a cation: * Collisions between atoms in a high temperature gas * Absorption of photon with sufficient energy to strip off an electron

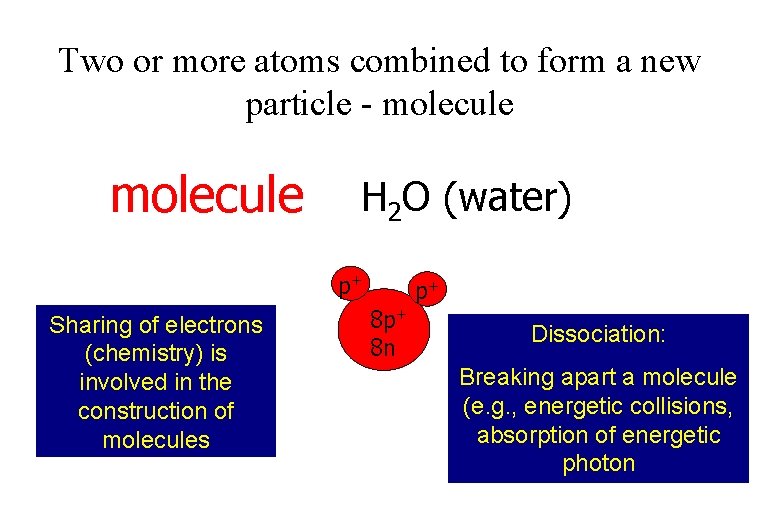
Two or more atoms combined to form a new particle - molecule H 2 O (water) p+ Sharing of electrons (chemistry) is involved in the construction of molecules 8 p+ 8 n p+ Dissociation: Breaking apart a molecule (e. g. , energetic collisions, absorption of energetic photon

Tritium is an unstable isotope of Hydrogen (1 p, 2 n) with a half life of 12. 3 yrs. If a sample of hydrogen initially has 1000 atoms of Tritium, how many will remain after 36. 9 yrs (two half-lives)? (yellow) 1000 (red) 500 (green) 250

Tritium is an unstable isotope of Hydrogen (1 p, 2 n) with a half life of 12. 3 yrs. If a sample of hydrogen initially has 1000 atoms of Tritium, how many will remain after 36. 9 yrs (two half-lives)? (yellow) 1000 (red) 500 (green) 250

If you added a proton to an atom to create a new stable, isolated atom, you would have created… (blue) an isotope of the original element (yellow) a fission reaction (red) a different element with a positive charge (green) a neutron and a positron

If you added a proton to an atom to create a new stable, isolated atom, you would have created… (blue) an isotope of the original element (yellow) a fission reaction (red) a different element with a positive charge (green) a neutron and a positron

If you removed an electron from an atom, you would have created (blue) an isotope of the original element (yellow) a fission reaction (red) a different element with a positive charge (green) an ionized atom

If you removed an electron from an atom, you would have created (blue) an isotope of the original element (yellow) a fission reaction (red) a different element with a positive charge (green) an ionized atom

If you combined two atoms such that they shared electrons to create a new stable object, you would have created (blue) an isotope of the original element (yellow) a molecule (red) a different element (green) an ionized atom

If you combined two atoms such that they shared electrons to create a new stable object, you would have created (blue) an isotope of the original element (yellow) a molecule (red) a different element (green) an ionized atom

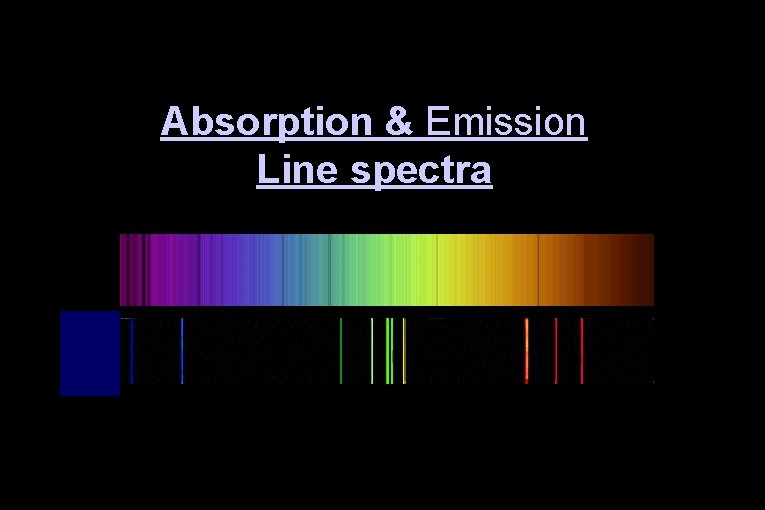
Absorption & Emission Line spectra

Electron Energy Levels ● ● ● Electrons cannot have just any energy while orbiting the nucleus. Only certain energy values are allowed (like the floors of an aprtment building). Electrons may only gain or lose certain specific amounts of energy (equal to differences in energy levels).

Electron Orbits / Absorption & Emission ● Electrons can gain or lose energy while they orbit the nucleus. ● When electrons have the lowest energy possible, we say the atom is in the ground state. ● When electrons have more energy than this, we say the atom is in an excited state. ● When electrons gain enough energy to escape the nucleus, we say the atom is ionized.

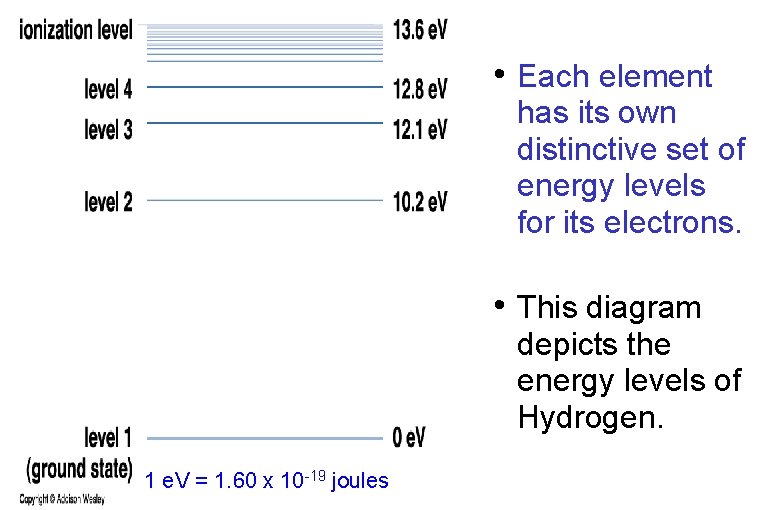
• Each element has its own distinctive set of energy levels for its electrons. • This diagram depicts the energy levels of Hydrogen. 1 e. V = 1. 60 x 10 -19 joules

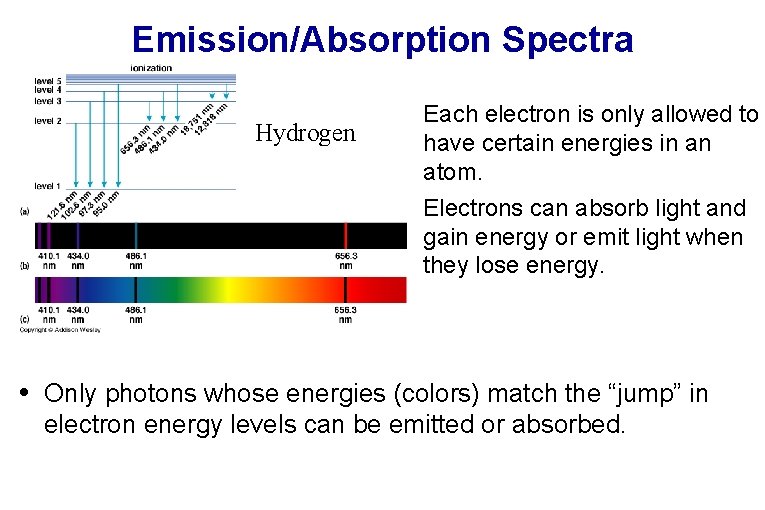
Emission/Absorption Spectra Hydrogen • Each electron is only allowed to have certain energies in an atom. • Electrons can absorb light and gain energy or emit light when they lose energy. • Only photons whose energies (colors) match the “jump” in electron energy levels can be emitted or absorbed.

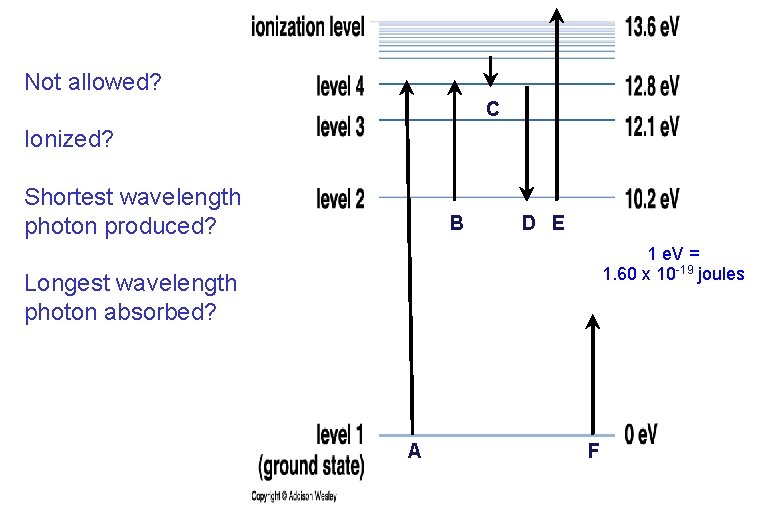
Not allowed? C Ionized? Shortest wavelength photon produced? B D E 1 e. V = 1. 60 x 10 -19 joules Longest wavelength photon absorbed? A F

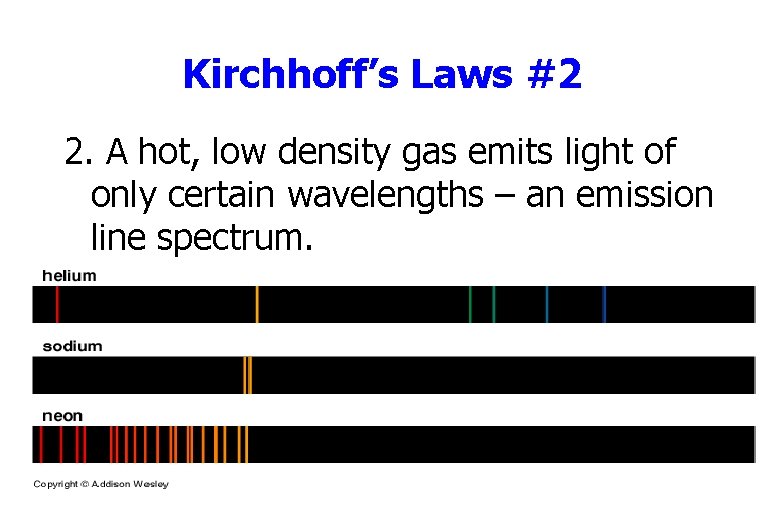
Kirchhoff’s Laws #2 2. A hot, low density gas emits light of only certain wavelengths – an emission line spectrum.



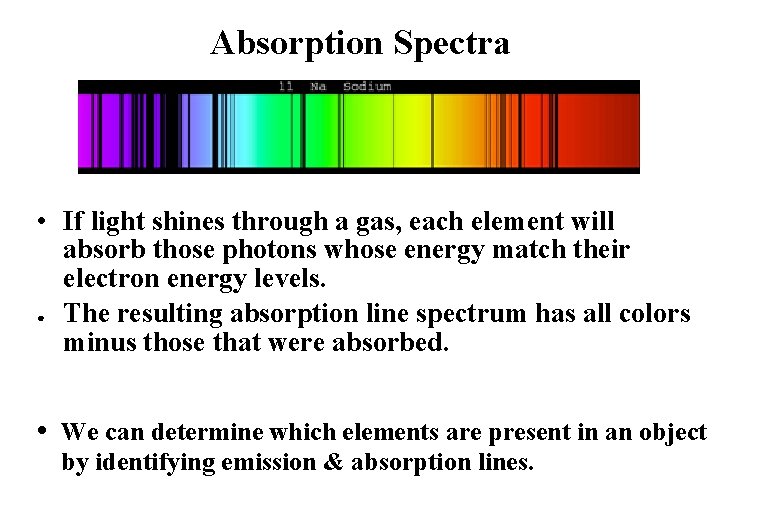
Absorption Spectra • If light shines through a gas, each element will absorb those photons whose energy match their electron energy levels. ● The resulting absorption line spectrum has all colors minus those that were absorbed. • We can determine which elements are present in an object by identifying emission & absorption lines.

Kirchhoff’s Law #3 3. When light having a continuous spectrum passes through a cool gas, dark lines appear in the continuous spectrum – an absorption line spectrum.


Molecules have rotational & vibrational energy levels – often showing spectral lines in the infrared and radio portion of the electromagnetic spectrum


Group Activity A look at different types of spectra, as predicted by Kirchhoff’s Laws Be sure to put your group name on the paper!!!

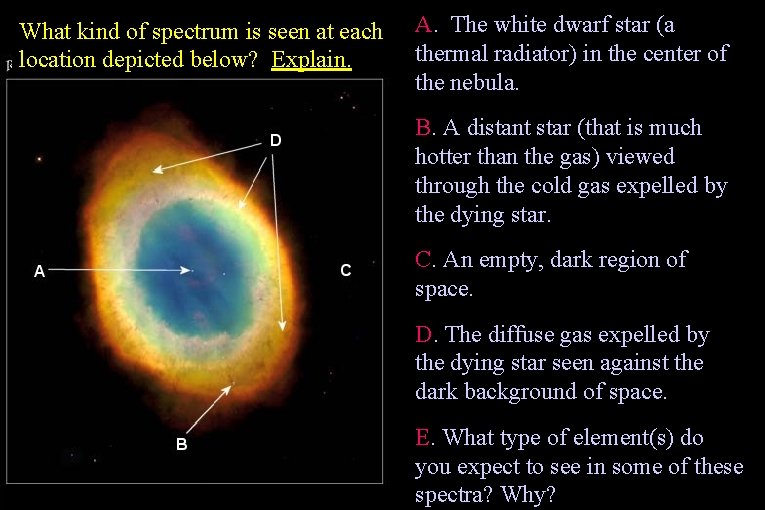
What kind of spectrum is seen at each location depicted below? Explain. A. The white dwarf star (a thermal radiator) in the center of the nebula. B. A distant star (that is much hotter than the gas) viewed through the cold gas expelled by the dying star. C. An empty, dark region of space. D. The diffuse gas expelled by the dying star seen against the dark background of space. E. What type of element(s) do you expect to see in some of these spectra? Why?