Its time to take care of TCell lymphomas



- Slides: 29

It’s time to take care of T-Cell lymphomas October 22 -24, 2006, Bologna, Italy Conventional Treatment of PTCL: The Asian Perspective Kensei Tobinai, MD, Ph. D National Cancer Center Hospital, Tokyo, Japan

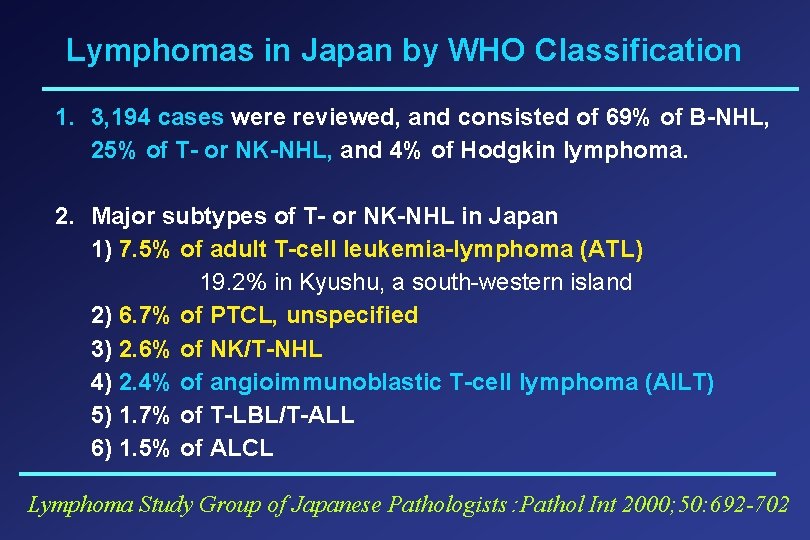
Topics of Today 1) Epidemiology of PTCL in Japan 2) JCOG Studies and Allogeneic SCT for ATL 3) CCR 4 Expression in ATL & PTCL-U and a Monoclonal Antibody Treatment 4) Concurrent Chemoradiotherapy of Nasal NK/T-NHL

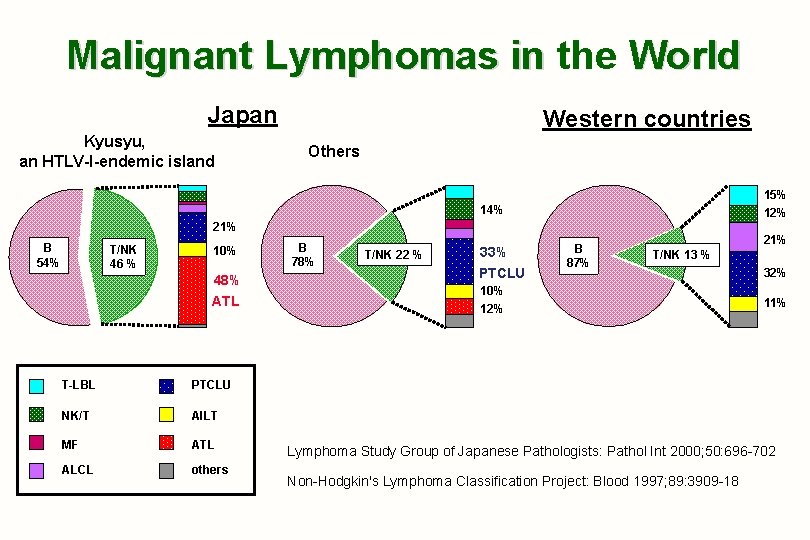
Lymphomas in Japan by WHO Classification 1. 3, 194 cases were reviewed, and consisted of 69% of B-NHL, 25% of T- or NK-NHL, and 4% of Hodgkin lymphoma. 2. Major subtypes of T- or NK-NHL in Japan 1) 7. 5% of adult T-cell leukemia-lymphoma (ATL) 19. 2% in Kyushu, a south-western island 2) 6. 7% of PTCL, unspecified 3) 2. 6% of NK/T-NHL 4) 2. 4% of angioimmunoblastic T-cell lymphoma (AILT) 5) 1. 7% of T-LBL/T-ALL 6) 1. 5% of ALCL Lymphoma Study Group of Japanese Pathologists:Pathol Int 2000; 50: 692 -702

Malignant Lymphomas in the World Japan Kyusyu, an HTLV-I-endemic island Western countries Others 15% 12% 14% 21% B 54% T/NK 46 % 10% 48% ATL T-LBL PTCLU NK/T AILT MF ATL ALCL others B 78% T/NK 22 % 33% PTCLU B 87% 21% T/NK 13 % 10% 12% 32% 11% Lymphoma Study Group of Japanese Pathologists: Pathol Int 2000; 50: 696 -702 Non-Hodgkin's Lymphoma Classification Project: Blood 1997; 89: 3909 -18

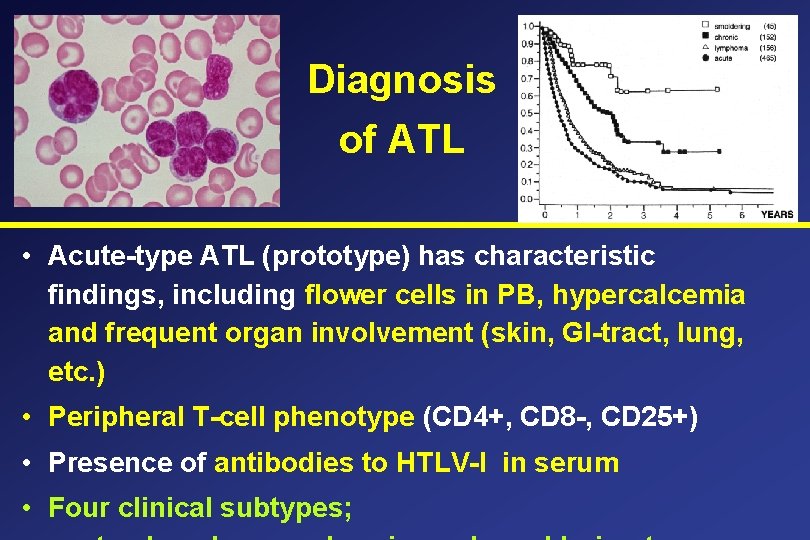
Diagnosis of ATL • Acute-type ATL (prototype) has characteristic findings, including flower cells in PB, hypercalcemia and frequent organ involvement (skin, GI-tract, lung, etc. ) • Peripheral T-cell phenotype (CD 4+, CD 8 -, CD 25+) • Presence of antibodies to HTLV-I in serum • Four clinical subtypes;

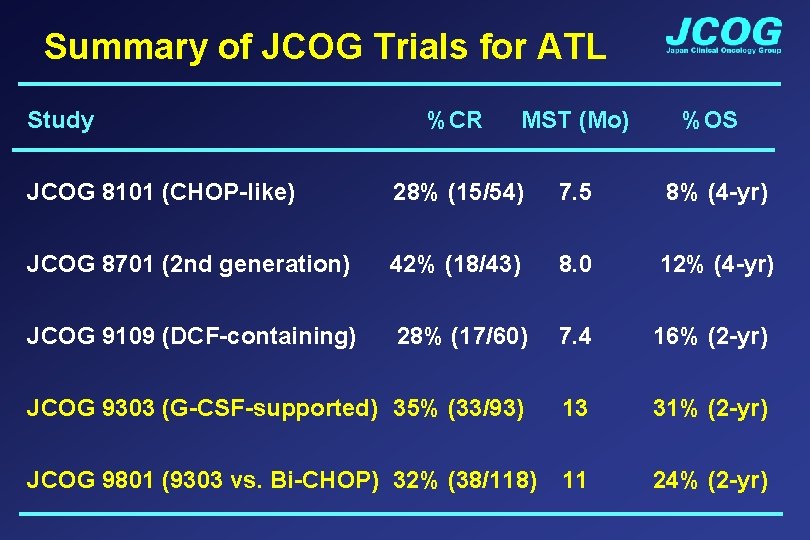
Summary of JCOG Trials for ATL Study %CR MST (Mo) %OS JCOG 8101 (CHOP-like) 28% (15/54) 7. 5 8% (4 -yr) JCOG 8701 (2 nd generation) 42% (18/43) 8. 0 12% (4 -yr) JCOG 9109 (DCF-containing) 28% (17/60) 7. 4 16% (2 -yr) JCOG 9303 (G-CSF-supported) 35% (33/93) 13 31% (2 -yr) JCOG 9801 (9303 vs. Bi-CHOP) 32% (38/118) 11 24% (2 -yr)

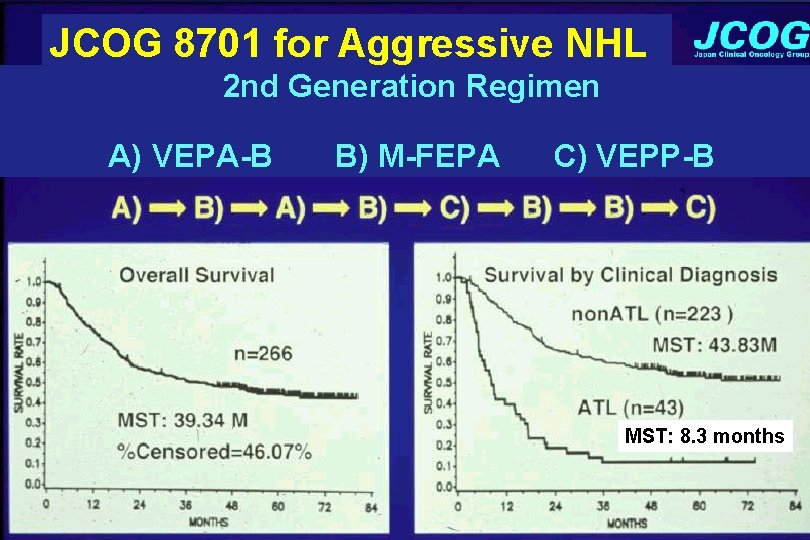
JCOG 8701 for Aggressive NHL 2 nd Generation Regimen A) VEPA-B B) M-FEPA C) VEPP-B MST: 8. 3 months

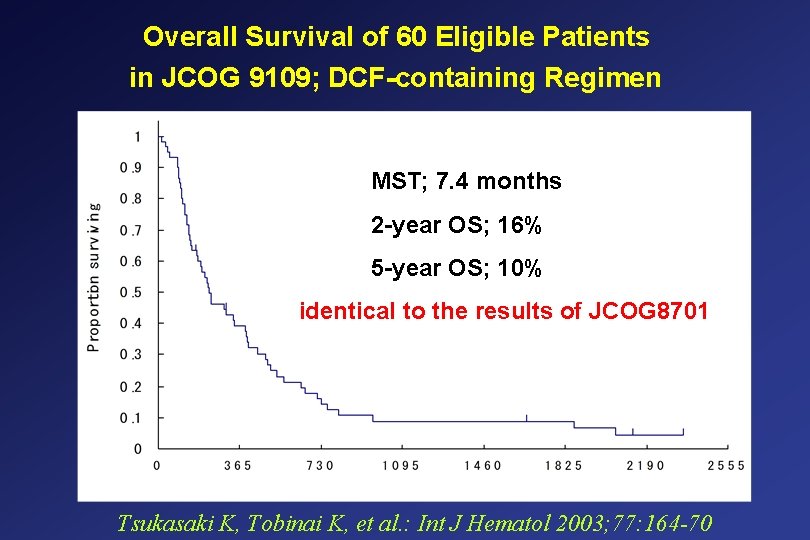
Overall Survival of 60 Eligible Patients in JCOG 9109; DCF-containing Regimen MST; 7. 4 months 2 -year OS; 16% 5 -year OS; 10% identical to the results of JCOG 8701 Tsukasaki K, Tobinai K, et al. : Int J Hematol 2003; 77: 164 -70

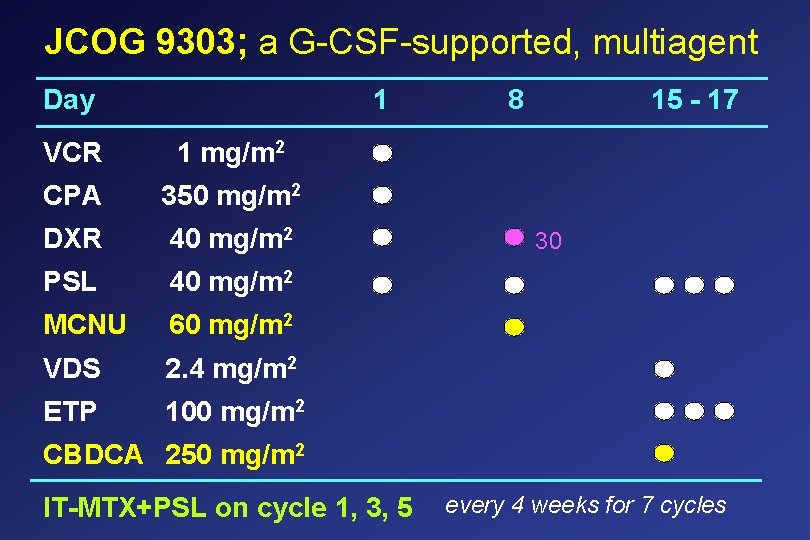
JCOG 9303; a G-CSF-supported, multiagent Day 1 VCR 1 mg/m 2 CPA 350 mg/m 2 DXR 40 mg/m 2 PSL 40 mg/m 2 MCNU 60 mg/m 2 VDS 2. 4 mg/m 2 ETP 100 mg/m 2 8 15 - 17 30 CBDCA 250 mg/m 2 IT-MTX+PSL on cycle 1, 3, 5 every 4 weeks for 7 cycles

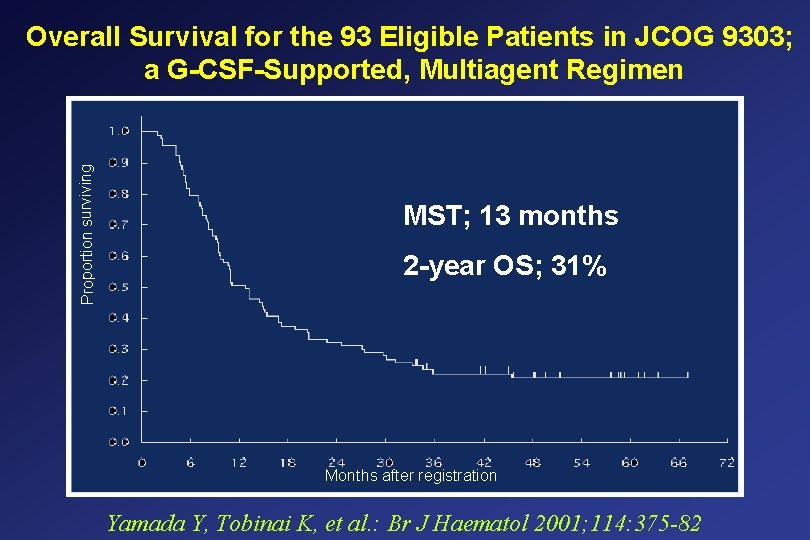
Proportion surviving Overall Survival for the 93 Eligible Patients in JCOG 9303; a G-CSF-Supported, Multiagent Regimen MST; 13 months 2 -year OS; 31% Months after registration Yamada Y, Tobinai K, et al. : Br J Haematol 2001; 114: 375 -82

Survival according to Clinical Subtypes in JCOG 9303 Hazard ratio; 1. 65 Lymphoma type MST; 19. 7 months Acute type MST; 10. 9 months Months after Registration Yamada Y, Tobinai K, et al. : Br J Haematol 2001; 114: 375 -82

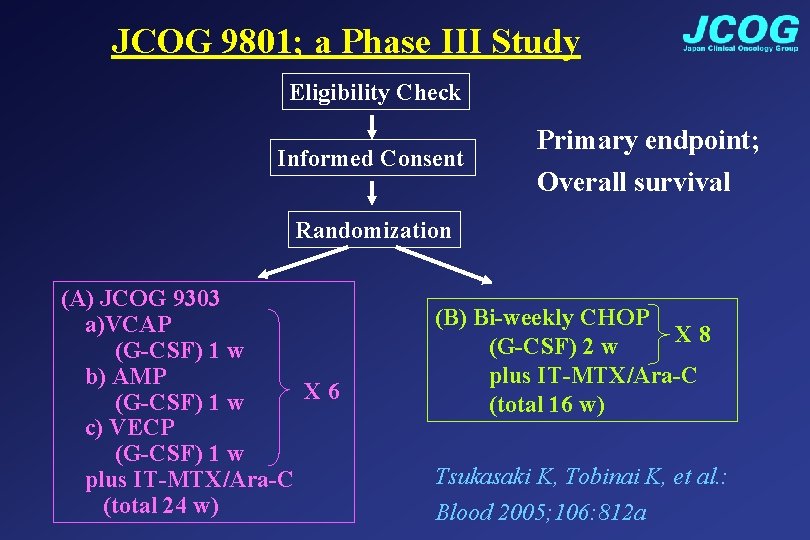
JCOG 9801; a Phase III Study Eligibility Check Informed Consent Primary endpoint; Overall survival Randomization (A) JCOG 9303 a)VCAP (G-CSF) 1 w b) AMP X 6 (G-CSF) 1 w c) VECP (G-CSF) 1 w plus IT-MTX/Ara-C (total 24 w) (B) Bi-weekly CHOP X 8 (G-CSF) 2 w plus IT-MTX/Ara-C (total 16 w) Tsukasaki K, Tobinai K, et al. : Blood 2005; 106: 812 a

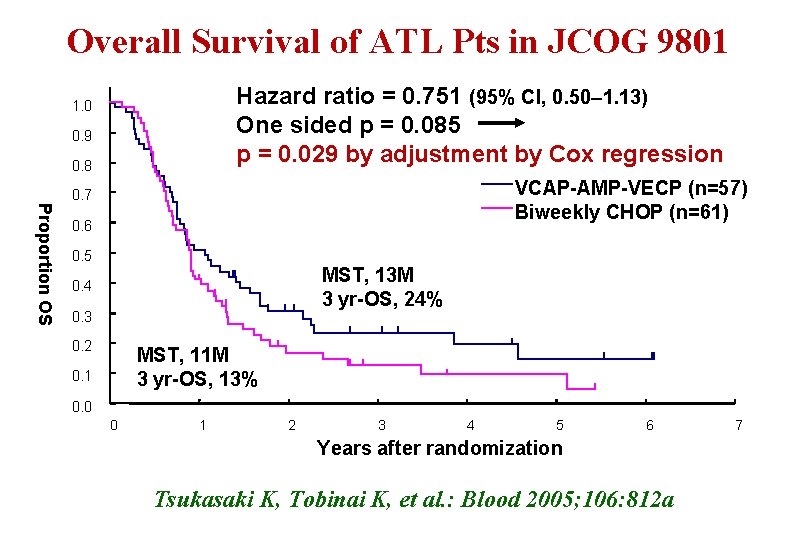
Overall Survival of ATL Pts in JCOG 9801 Hazard ratio = 0. 751 (95% CI, 0. 50– 1. 13) One sided p = 0. 085 p = 0. 029 by adjustment by Cox regression 1. 0 0. 9 0. 8 Proportion OS VCAP-AMP-VECP (n=57) Biweekly CHOP (n=61) 0. 7 0. 6 0. 5 MST, 13 M 3 yr-OS, 24% 0. 4 0. 3 0. 2 MST, 11 M 3 yr-OS, 13% 0. 1 0. 0 0 1 2 3 4 5 6 Years after randomization Tsukasaki K, Tobinai K, et al. : Blood 2005; 106: 812 a 7

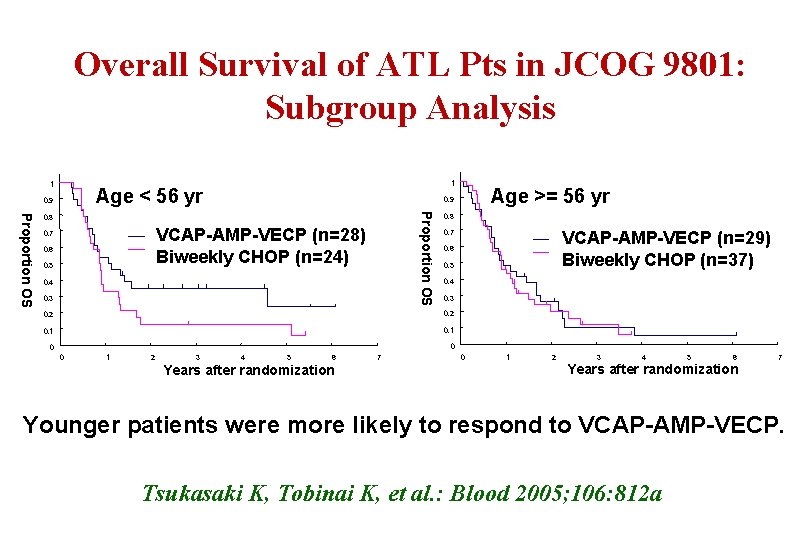
Overall Survival of ATL Pts in JCOG 9801: Subgroup Analysis 1 1 Age < 56 yr 0. 9 Proportion OS 0. 8 VCAP-AMP-VECP (n=28) Biweekly CHOP (n=24) 0. 7 0. 6 0. 5 Age >= 56 yr 0. 9 0. 4 0. 3 0. 8 0. 7 VCAP-AMP-VECP (n=29) Biweekly CHOP (n=37) 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0 0 0 1 2 3 4 5 6 Years after randomization 7 0 1 2 3 4 5 6 7 Years after randomization Younger patients were more likely to respond to VCAP-AMP-VECP. Tsukasaki K, Tobinai K, et al. : Blood 2005; 106: 812 a

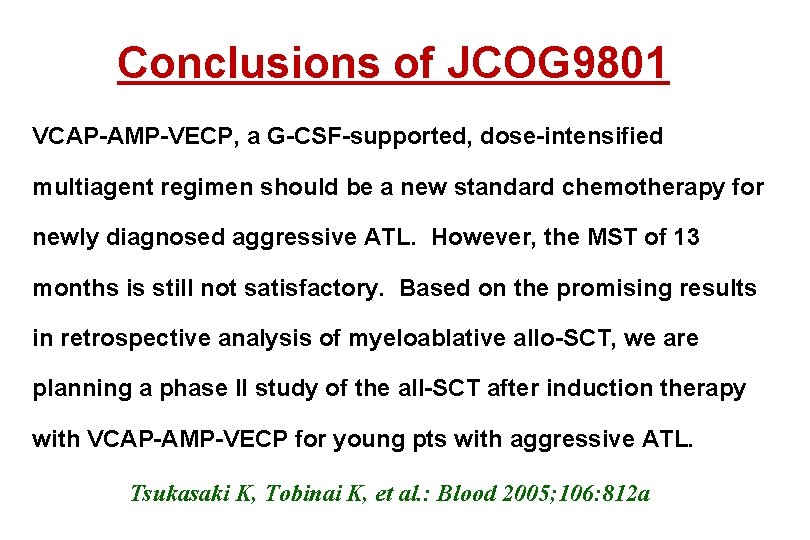
Conclusions of JCOG 9801 VCAP-AMP-VECP, a G-CSF-supported, dose-intensified multiagent regimen should be a new standard chemotherapy for newly diagnosed aggressive ATL. However, the MST of 13 months is still not satisfactory. Based on the promising results in retrospective analysis of myeloablative allo-SCT, we are planning a phase II study of the all-SCT after induction therapy with VCAP-AMP-VECP for young pts with aggressive ATL. Tsukasaki K, Tobinai K, et al. : Blood 2005; 106: 812 a

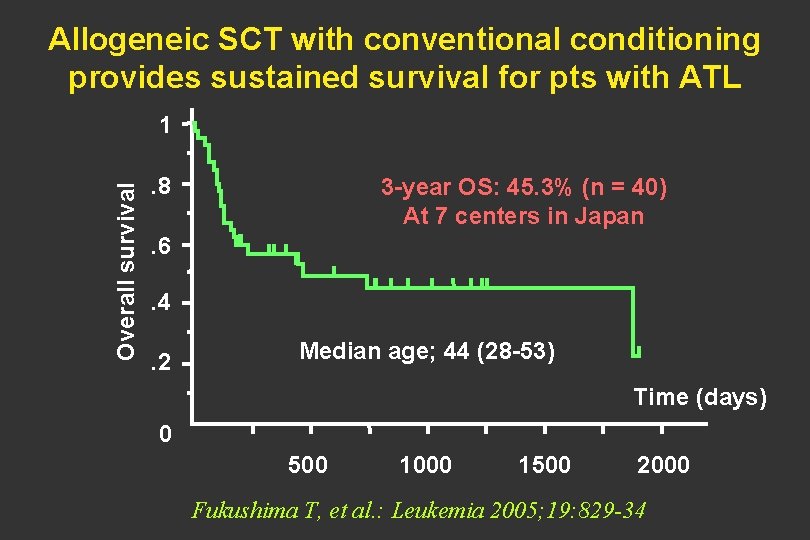
Allogeneic SCT with conventional conditioning provides sustained survival for pts with ATL Overall survival 1. 8 3 -year OS: 45. 3% (n = 40) At 7 centers in Japan . 6. 4. 2 Median age; 44 (28 -53) Time (days) 0 500 1000 1500 2000 Fukushima T, et al. : Leukemia 2005; 19: 829 -34

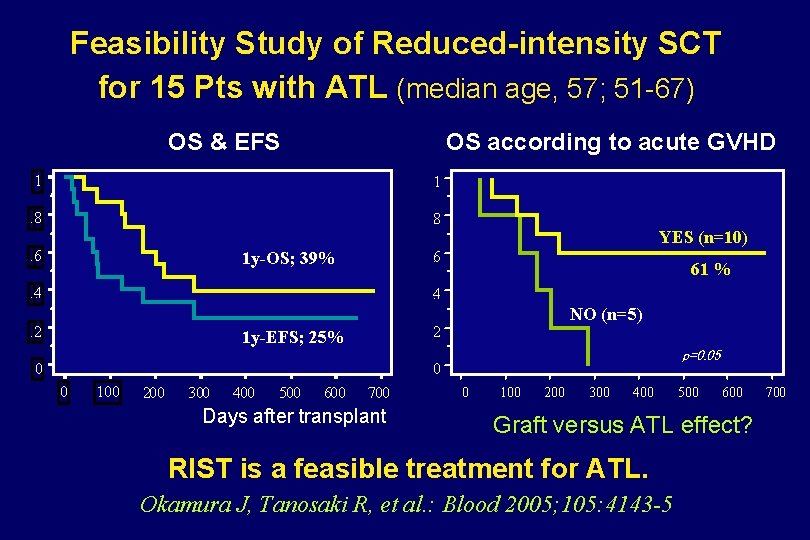
Feasibility Study of Reduced-intensity SCT for 15 Pts with ATL (median age, 57; 51 -67) OS & EFS OS according to acute GVHD 1 1 . 8. . 6. 1 y-OS; 39% YES (n=10) 6. . 4. 61 % 4. . 2. NO (n=5) 2. 1 y-EFS; 25% 0 p=0. 05 0 0 100 200 300 400 500 600 700 Days after transplant 0 100 200 300 400 500 600 Graft versus ATL effect? RIST is a feasible treatment for ATL. Okamura J, Tanosaki R, et al. : Blood 2005; 105: 4143 -5 700

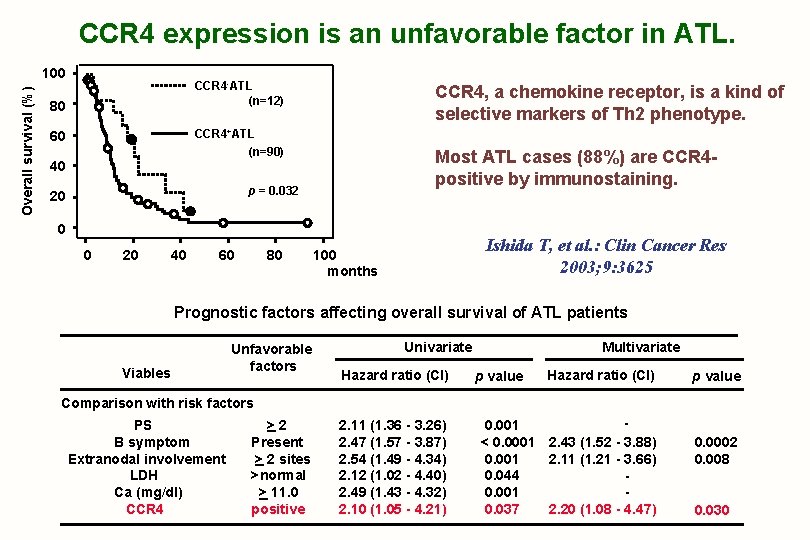
CCR 4 expression is an unfavorable factor in ATL. Overall survival (%) 100 CCR 4 -ATL (n=12) 80 CCR 4, a chemokine receptor, is a kind of selective markers of Th 2 phenotype. CCR 4+ATL 60 (n=90) 40 Most ATL cases (88%) are CCR 4 positive by immunostaining. p = 0. 032 20 0 0 20 40 60 80 Ishida T, et al. : Clin Cancer Res 2003; 9: 3625 100 months Prognostic factors affecting overall survival of ATL patients Viables Unfavorable factors Univariate Hazard ratio (CI) Multivariate p value Hazard ratio (CI) p value Comparison with risk factors PS B symptom Extranodal involvement LDH Ca (mg/dl) CCR 4 >2 Present > 2 sites >normal > 11. 0 positive 2. 11 (1. 36 - 3. 26) 2. 47 (1. 57 - 3. 87) 2. 54 (1. 49 - 4. 34) 2. 12 (1. 02 - 4. 40) 2. 49 (1. 43 - 4. 32) 2. 10 (1. 05 - 4. 21) 0. 001 < 0. 0001 2. 43 (1. 52 - 3. 88) 0. 001 2. 11 (1. 21 - 3. 66) 0. 044 0. 001 0. 037 2. 20 (1. 08 - 4. 47) 0. 0002 0. 008 0. 030

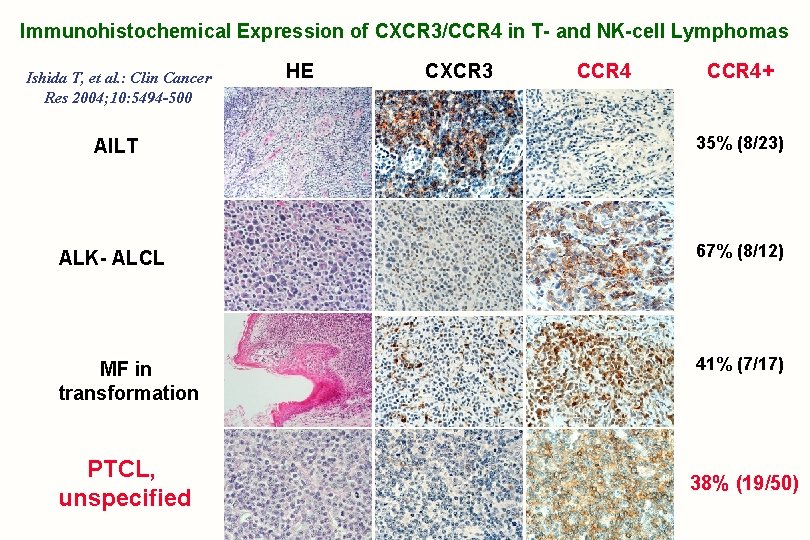
Immunohistochemical Expression of CXCR 3/CCR 4 in T- and NK-cell Lymphomas Ishida T, et al. : Clin Cancer Res 2004; 10: 5494 -500 HE CXCR 3 CCR 4+ AILT 35% (8/23) ALK- ALCL 67% (8/12) MF in transformation 41% (7/17) PTCL, unspecified 38% (19/50)

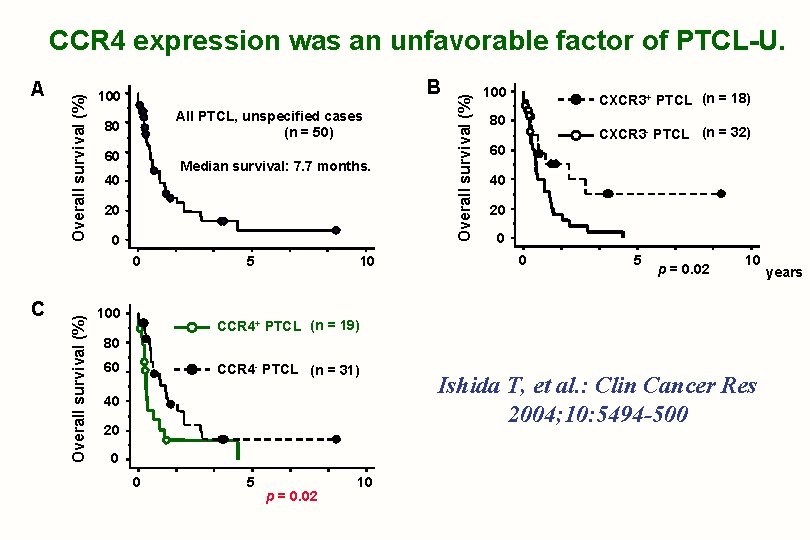
B 100 All PTCL, unspecified cases (n = 50) 80 60 Median survival: 7. 7 months. 40 20 0 C Overall survival (%) 0 100 5 10 Overall survival (%) A Overall survival (%) CCR 4 expression was an unfavorable factor of PTCL-U. 100 CXCR 3+ PTCL (n = 18) 80 CXCR 3 - PTCL (n = 32) 60 40 20 0 0 5 p = 0. 02 10 CCR 4+ PTCL (n = 19) 80 60 CCR 4 - PTCL (n = 31) 40 20 0 0 5 p = 0. 02 10 Ishida T, et al. : Clin Cancer Res 2004; 10: 5494 -500 years

Chemokine Receptors & PTCL 1) Chemokine receptors may be useful not only for further characterization of T- and NK-cell lymphomas, but also in predicting clinical outcomes. 2) Most ATL cases (88%) are CCR 4 -positive by immunostaining. CCR 4 expression is an unfavorable prognostic factor in ATL and PTCL-U. Ishida T, et al. : Clin Cancer Res 2004; 10: 5494 -500

Phase I Study of an ADCC-Enhanced, Humanized Anti-CCR 4 Antibody (KW-0761) in Patients with CCR 4 Positive Peripheral T-Cell Malignancies Pre-clinical studies of KW-0761 showed promising results for its clinical application to T-cell malignancies, especially to ATL. Patient enrolment will be initiated very soon in Japan.

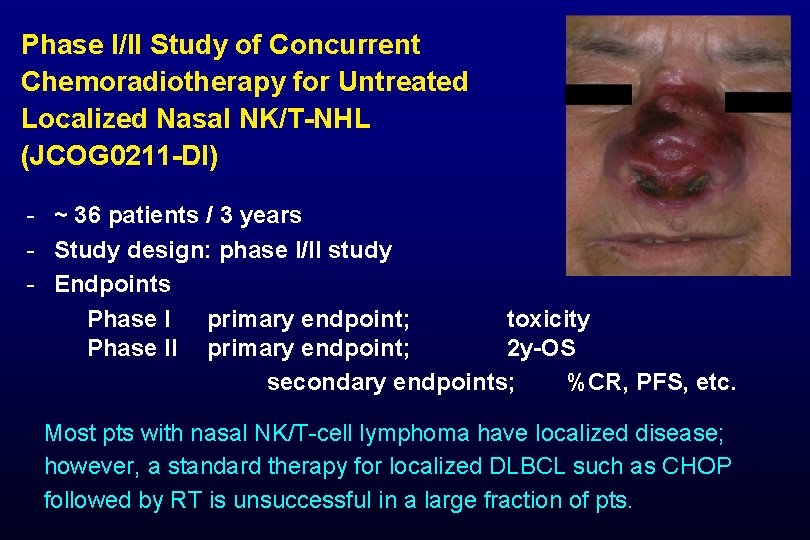
Phase I/II Study of Concurrent Chemoradiotherapy for Untreated Localized Nasal NK/T-NHL (JCOG 0211 -DI) - ~ 36 patients / 3 years - Study design: phase I/II study - Endpoints Phase I primary endpoint; toxicity Phase II primary endpoint; 2 y-OS secondary endpoints; %CR, PFS, etc. Most pts with nasal NK/T-cell lymphoma have localized disease; however, a standard therapy for localized DLBCL such as CHOP followed by RT is unsuccessful in a large fraction of pts.

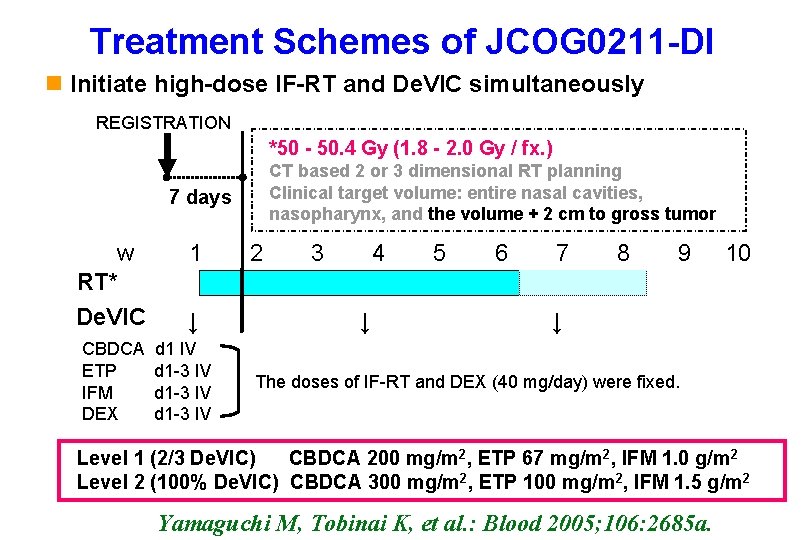
Treatment Schemes of JCOG 0211 -DI n Initiate high-dose IF-RT and De. VIC simultaneously REGISTRATION *50 - 50. 4 Gy (1. 8 - 2. 0 Gy / fx. ) 7 days w RT* De. VIC CBDCA ETP IFM DEX CT based 2 or 3 dimensional RT planning Clinical target volume: entire nasal cavities, nasopharynx, and the volume + 2 cm to gross tumor 1 2 3 4 5 6 7 8 9 ↓ ↓ d 1 IV d 1 -3 IV 10 ↓ The doses of IF-RT and DEX (40 mg/day) were fixed. Level 1 (2/3 De. VIC) CBDCA 200 mg/m 2, ETP 67 mg/m 2, IFM 1. 0 g/m 2 Level 2 (100% De. VIC) CBDCA 300 mg/m 2, ETP 100 mg/m 2, IFM 1. 5 g/m 2 Hematology Division, National Cancer Center Yamaguchi M, Tobinai K, et al. : Blood 2005; 106: 2685 a.

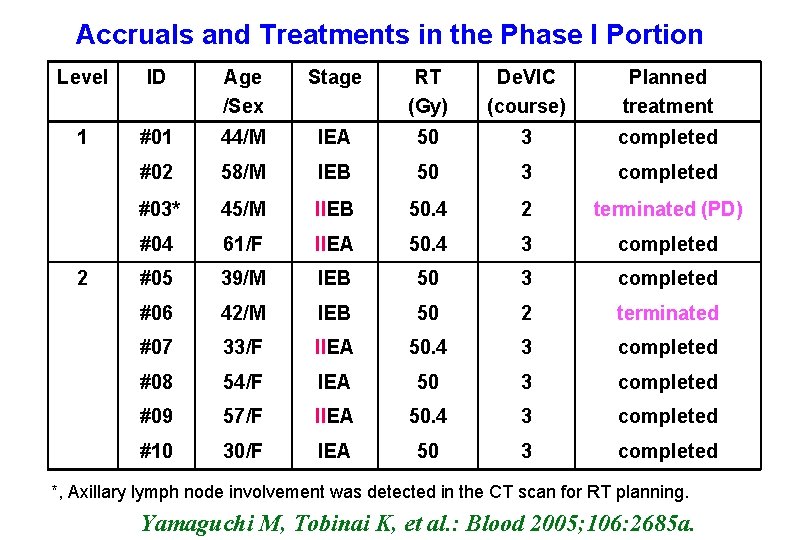
Accruals and Treatments in the Phase I Portion Level ID Age /Sex Stage RT (Gy) De. VIC (course) Planned treatment 1 #01 44/M IEA 50 3 completed #02 58/M IEB 50 3 completed #03* 45/M IIEB 50. 4 2 terminated (PD) #04 61/F IIEA 50. 4 3 completed #05 39/M IEB 50 3 completed #06 42/M IEB 50 2 terminated #07 33/F IIEA 50. 4 3 completed #08 54/F IEA 50 3 completed #09 57/F IIEA 50. 4 3 completed #10 30/F IEA 50 3 completed 2 *, Axillary lymph node involvement was detected in the CT scan for RT planning. Yamaguchi M, Tobinai K, et al. : Blood 2005; 106: 2685 a.

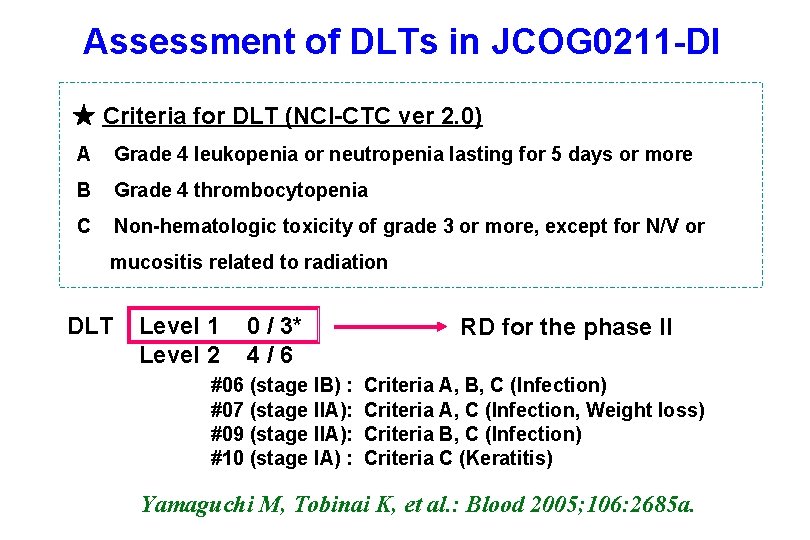
Assessment of DLTs in JCOG 0211 -DI ★ Criteria for DLT (NCI-CTC ver 2. 0) A Grade 4 leukopenia or neutropenia lasting for 5 days or more B Grade 4 thrombocytopenia C Non-hematologic toxicity of grade 3 or more, except for N/V or mucositis related to radiation DLT Level 1 Level 2 0 / 3* 4 / 6 #06 (stage IB) : #07 (stage IIA): #09 (stage IIA): #10 (stage IA) : RD for the phase II Criteria A, B, C (Infection) Criteria A, C (Infection, Weight loss) Criteria B, C (Infection) Criteria C (Keratitis) Yamaguchi M, Tobinai K, et al. : Blood 2005; 106: 2685 a.

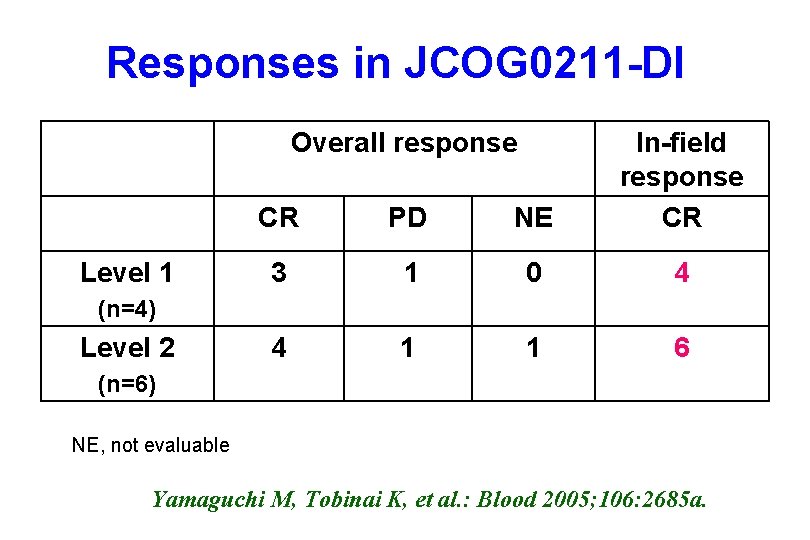
Responses in JCOG 0211 -DI Overall response Level 1 CR PD NE In-field response CR 3 1 0 4 4 1 1 6 (n=4) Level 2 (n=6) NE, not evaluable Yamaguchi M, Tobinai K, et al. : Blood 2005; 106: 2685 a.

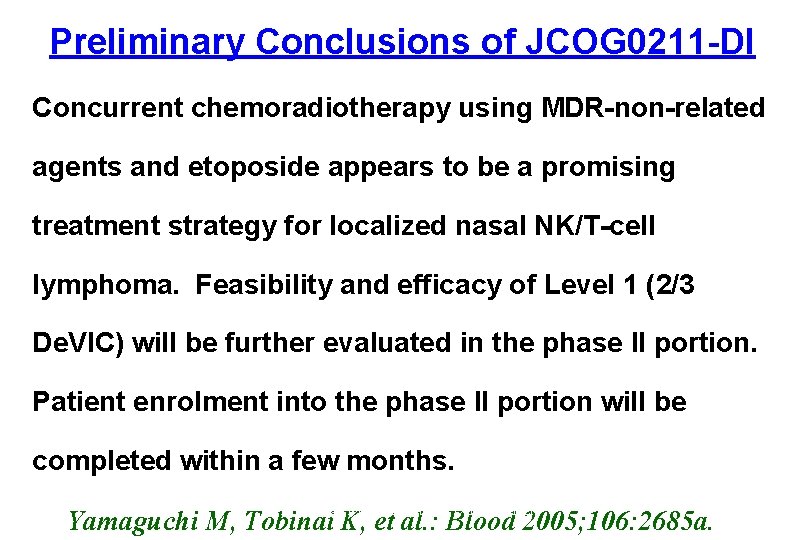
Preliminary Conclusions of JCOG 0211 -DI Concurrent chemoradiotherapy using MDR-non-related agents and etoposide appears to be a promising treatment strategy for localized nasal NK/T-cell lymphoma. Feasibility and efficacy of Level 1 (2/3 De. VIC) will be further evaluated in the phase II portion. Patient enrolment into the phase II portion will be completed within a few months. Hematology Division, National Cancer Center Yamaguchi M, Tobinai K, et al. : Blood 2005; 106: 2685 a.

Acknowledgements • JCOG studies for ATL Tsukasaki K, Yamada Y, Fukuda H, Shimoyama M, et al. • Allogeneic SCT for ATL Fukushima T, Okamura J, et al. • CCR 4 expression in ATL & PTCL-U Ishida T, Ueda R, et al. • Phase I/II study for nasal NK/T-NHL Yamaguchi M, Oguchi M, et al.
 Primary secondary tertiary care
Primary secondary tertiary care Take a bus or take a train
Take a bus or take a train For minutes. start.
For minutes. start. Leadership personal statement examples
Leadership personal statement examples Drake rihanna take care traduction
Drake rihanna take care traduction How to take care of your nervous system
How to take care of your nervous system How to care for the skeletal system
How to care for the skeletal system Rsa take care
Rsa take care Rsa take care
Rsa take care Take care dalam islam
Take care dalam islam Hello take care
Hello take care You 1
You 1 Pls take care of yourself
Pls take care of yourself Youhol
Youhol Take care of sense organs
Take care of sense organs The graceful slopes glow even clearer
The graceful slopes glow even clearer When a train increases its velocity its momentum
When a train increases its velocity its momentum Sunny cloudy rainy snowy windy
Sunny cloudy rainy snowy windy If its square its a sonnet summary
If its square its a sonnet summary Its halloween its halloween the moon is full and bright
Its halloween its halloween the moon is full and bright Its not easy but its worth it
Its not easy but its worth it Ipde definition
Ipde definition Please take out some time
Please take out some time Duty of care outcome care certificate
Duty of care outcome care certificate Magnetul atrage
Magnetul atrage Palliative care versus hospice care
Palliative care versus hospice care Nasc pui vii
Nasc pui vii Care sunt simturile prin care sunt evocate
Care sunt simturile prin care sunt evocate Standard 3 care certificate answers
Standard 3 care certificate answers Hip fracture clinical care standard
Hip fracture clinical care standard