PERIPHERAL TCELL LYMPHOMAS HOW I MANAGE PTCL IN






- Slides: 63

PERIPHERAL T-CELL LYMPHOMAS: HOW I MANAGE PTCL IN THE FRONTLINE SETTING Owen A. O’Connor, M. D. , Ph. D. owenoconnor@columbia. edu Professor of Medicine and Experimental Therapeutics Director, Center for Lymphoid Malignancies Columbia University Medical Center – College of Physicians and Surgeons The New York Presbyterian Hospital New York, N. Y. Center for Lymphoid Malignancies

PERIPHERAL T-CELL LYMPHOMAS: HOW I MANAGE FRONTLINE DISEASE · Putting The T-cell Lymphomas in Context · What Are the Optimal Upfront Treatment Considerations · Bridging Patients with Relapsed or Refractory Disease with Novel Drugs · Conclusion

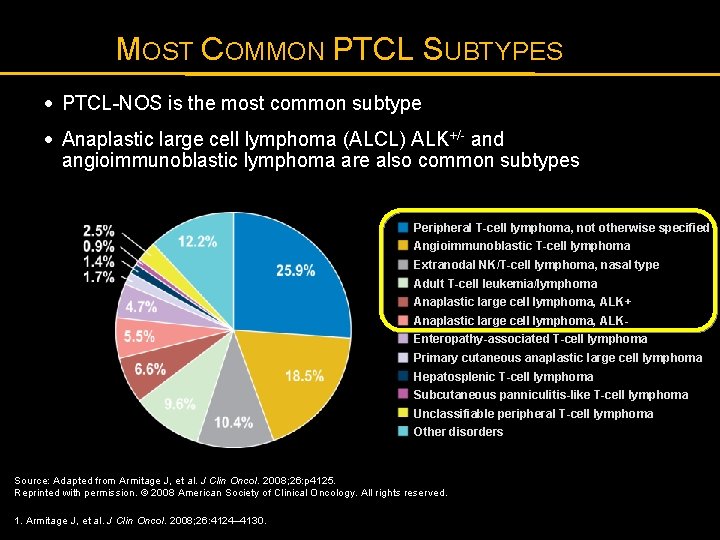
MOST COMMON PTCL SUBTYPES · PTCL-NOS is the most common subtype · Anaplastic large cell lymphoma (ALCL) ALK+/- and angioimmunoblastic lymphoma are also common subtypes Peripheral T-cell lymphoma, not otherwise specified Angioimmunoblastic T-cell lymphoma Extranodal NK/T-cell lymphoma, nasal type Adult T-cell leukemia/lymphoma Anaplastic large cell lymphoma, ALK+ Anaplastic large cell lymphoma, ALKEnteropathy-associated T-cell lymphoma Primary cutaneous anaplastic large cell lymphoma Hepatosplenic T-cell lymphoma Subcutaneous panniculitis-like T-cell lymphoma Unclassifiable peripheral T-cell lymphoma Other disorders Source: Adapted from Armitage J, et al. J Clin Oncol. 2008; 26: p 4125. Reprinted with permission. © 2008 American Society of Clinical Oncology. All rights reserved. 1. Armitage J, et al. J Clin Oncol. 2008; 26: 4124– 4130.

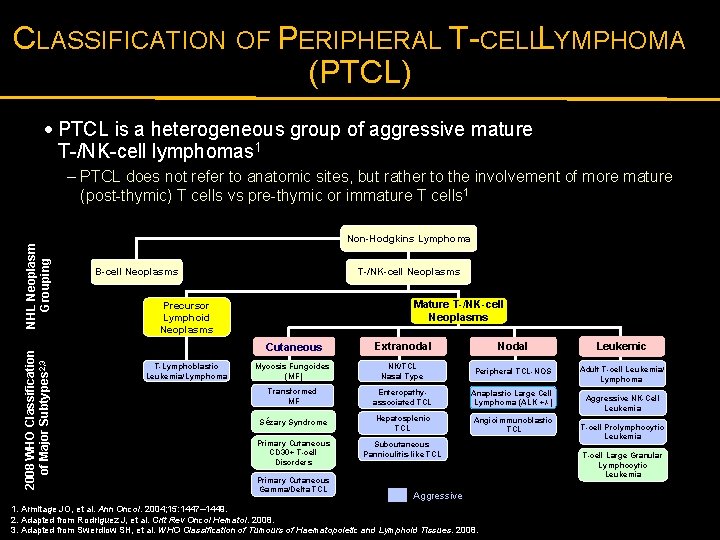
CLASSIFICATION OF PERIPHERAL T-CELLL YMPHOMA (PTCL) · PTCL is a heterogeneous group of aggressive mature T-/NK-cell lymphomas 1 2008 WHO Classification of Major Subtypes 2, 3 NHL Neoplasm Grouping – PTCL does not refer to anatomic sites, but rather to the involvement of more mature (post-thymic) T cells vs pre-thymic or immature T cells 1 Non-Hodgkins Lymphoma T-/NK-cell Neoplasms B-cell Neoplasms Mature T-/NK-cell Neoplasms Precursor Lymphoid Neoplasms T-Lymphoblastic Leukemia/Lymphoma Cutaneous Extranodal Nodal Leukemic Mycosis Fungoides (MF) NK/TCL Nasal Type Peripheral TCL-NOS Adult T-cell Leukemia/ Lymphoma Transformed MF Enteropathyassociated TCL Anaplastic Large Cell Lymphoma (ALK +/-) Sézary Syndrome Hepatosplenic TCL Angioimmunoblastic TCL Primary Cutaneous CD 30+ T-cell Disorders Subcutaneous Panniculitis-like TCL Primary Cutaneous Gamma/Delta TCL Aggressive 1. Armitage JO, et al. Ann Oncol. 2004; 15: 1447– 1449. 2. Adapted from Rodriguez J, et al. Crit Rev Oncol Hematol. 2008. 3. Adapted from Swerdlow SH, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 2008. Aggressive NK-Cell Leukemia T-cell Prolymphocytic Leukemia T-cell Large Granular Lymphocytic Leukemia

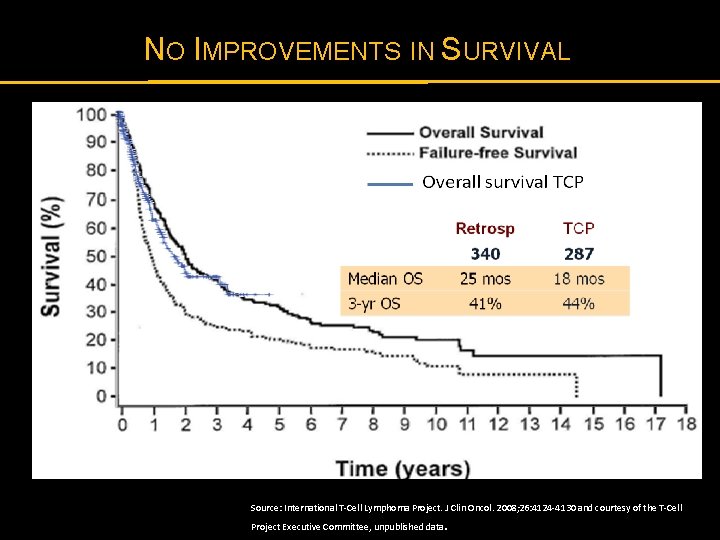
NO IMPROVEMENTS IN SURVIVAL Source: International T-Cell Lymphoma Project. J Clin Oncol. 2008; 26: 4124 -4130 and courtesy of the T-Cell Project Executive Committee, unpublished data .

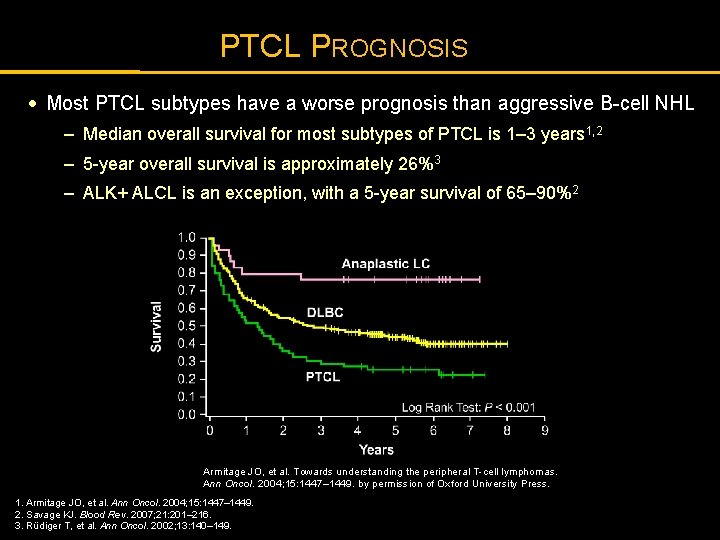
PTCL PROGNOSIS · Most PTCL subtypes have a worse prognosis than aggressive B-cell NHL – Median overall survival for most subtypes of PTCL is 1– 3 years 1, 2 – 5 -year overall survival is approximately 26%3 – ALK+ ALCL is an exception, with a 5 -year survival of 65– 90%2 Armitage JO, et al. Towards understanding the peripheral T-cell lymphomas. Ann Oncol. 2004; 15: 1447– 1449. by permission of Oxford University Press. 1. Armitage JO, et al. Ann Oncol. 2004; 15: 1447– 1449. 2. Savage KJ. Blood Rev. 2007; 21: 201– 216. 3. Rüdiger T, et al. Ann Oncol. 2002; 13: 140– 149.

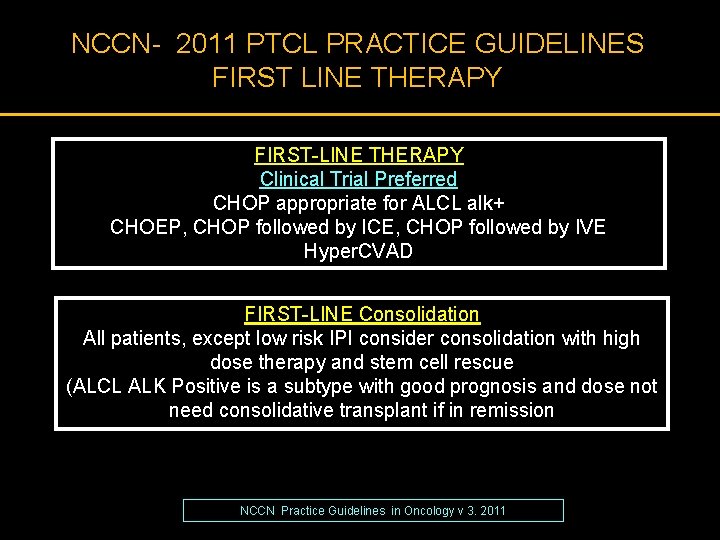
NCCN- 2011 PTCL PRACTICE GUIDELINES FIRST LINE THERAPY FIRST-LINE THERAPY Clinical Trial Preferred CHOP appropriate for ALCL alk+ CHOEP, CHOP followed by ICE, CHOP followed by IVE Hyper. CVAD FIRST-LINE Consolidation All patients, except low risk IPI consider consolidation with high dose therapy and stem cell rescue (ALCL ALK Positive is a subtype with good prognosis and dose not need consolidative transplant if in remission NCCN Practice Guidelines in Oncology v 3. 2011

PERIPHERAL T-CELL LYMPHOMAS: HOW I MANAGE FRONTLINE DISEASE · Putting The T-cell Lymphomas in Context · What Are the Optimal Upfront Treatment Considerations · Bridging Patients with Relapsed or Refractory Disease with Novel Drugs · Conclusion

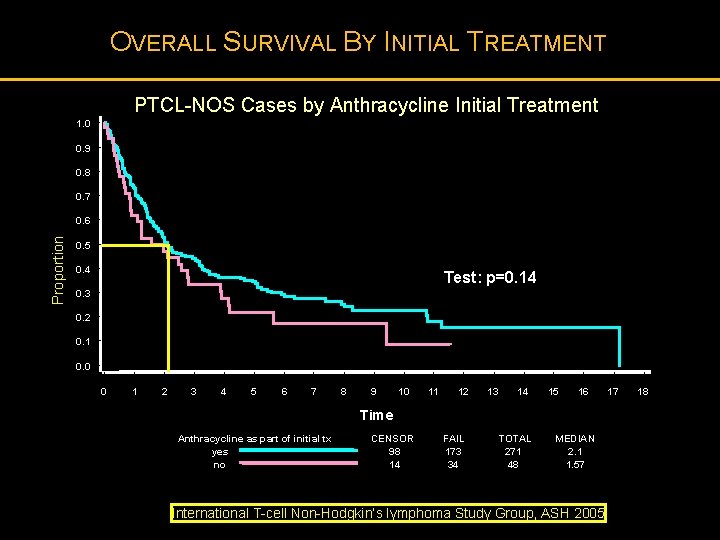
OVERALL SURVIVAL BY INITIAL TREATMENT PTCL-NOS Cases by Anthracycline Initial Treatment 1. 0 0. 9 0. 8 0. 7 Proportion 0. 6 0. 5 0. 4 Test: p=0. 14 0. 3 0. 2 0. 1 0. 0 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Time Anthracycline as part of initial tx yes no CENSOR 98 14 FAIL 173 34 TOTAL 271 48 MEDIAN 2. 1 1. 57 International T-cell Non-Hodgkin’s lymphoma Study Group, ASH 2005 17 18

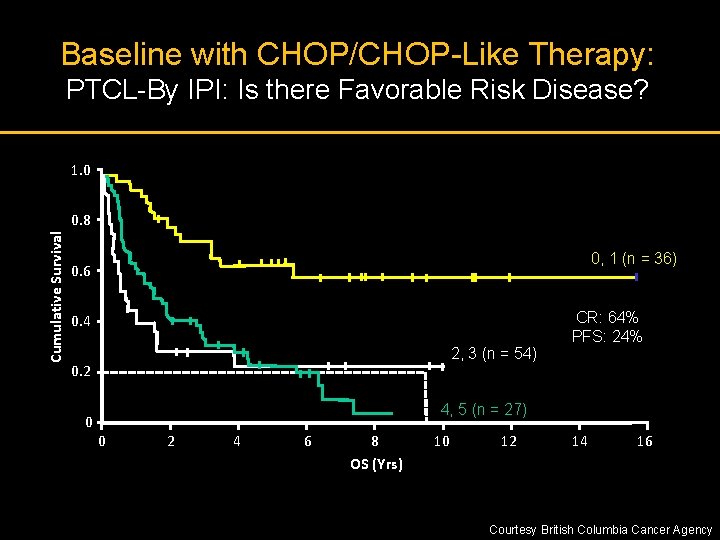
Baseline with CHOP/CHOP-Like Therapy: PTCL-By IPI: Is there Favorable Risk Disease? 1. 0 Cumulative Survival 0. 8 0, 1 (n = 36) 0. 6 0. 4 2, 3 (n = 54) 0. 2 0 CR: 64% PFS: 24% 4, 5 (n = 27) 0 2 4 6 8 OS (Yrs) 10 12 14 16 Courtesy British Columbia Cancer Agency

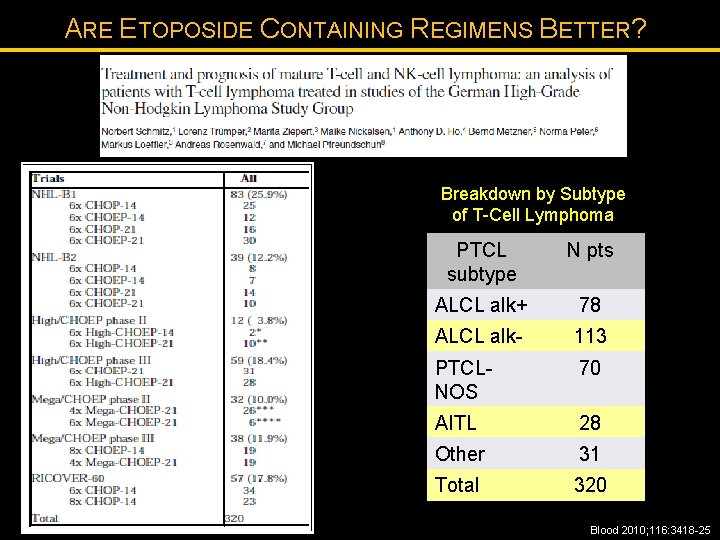
ARE ETOPOSIDE CONTAINING REGIMENS BETTER? Breakdown by Subtype of T-Cell Lymphoma PTCL subtype N pts ALCL alk+ 78 ALCL alk- 113 PTCLNOS 70 AITL 28 Other 31 Total 320 Blood 2010; 116: 3418 -25

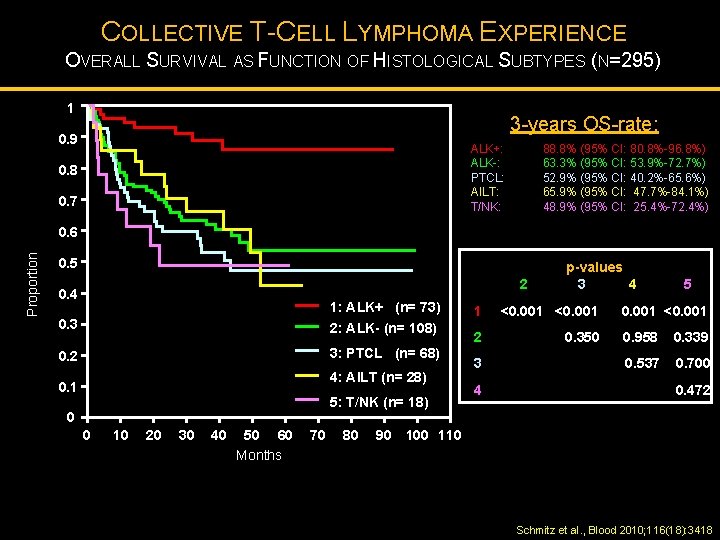
COLLECTIVE T-CELL LYMPHOMA EXPERIENCE OVERALL SURVIVAL AS FUNCTION OF HISTOLOGICAL SUBTYPES (N=295) 1 3 -years OS-rate: 0. 9 0. 8 ALK+: ALK-: PTCL: AILT: T/NK: 0. 7 88. 8% (95% CI: 80. 8%-96. 8%) 63. 3% (95% CI: 53. 9%-72. 7%) 52. 9% (95% CI: 40. 2%-65. 6%) 65. 9% (95% CI: 47. 7%-84. 1%) 48. 9% (95% CI: 25. 4%-72. 4%) Proportion 0. 6 0. 5 2 0. 4 1: ALK+ (n= 73) 0. 3 2: ALK- (n= 108) 0. 2 3: PTCL (n= 68) 4: AILT (n= 28) 0. 1 5: T/NK (n= 18) 0 0 10 20 30 40 50 60 Months 70 80 90 p-values 3 4 1 <0. 001 2 0. 350 3 4 5 0. 001 <0. 001 0. 958 0. 339 0. 537 0. 700 0. 472 100 110 Schmitz et al. , Blood 2010; 116(18): 3418

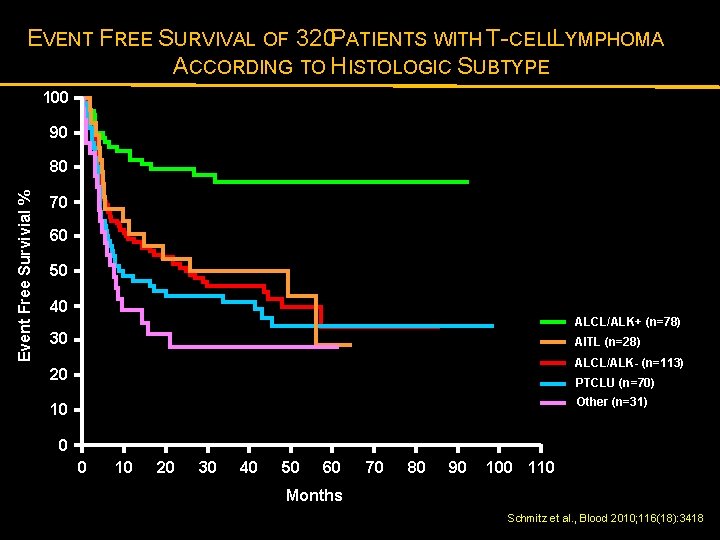
EVENT FREE SURVIVAL OF 320 PATIENTS WITH T-CELLL YMPHOMA ACCORDING TO HISTOLOGIC SUBTYPE 100 90 Event Free Survivial % 80 70 60 50 40 ALCL/ALK+ (n=78) 30 AITL (n=28) ALCL/ALK- (n=113) 20 PTCLU (n=70) Other (n=31) 10 0 0 10 20 30 40 50 60 70 80 90 100 110 Months Schmitz et al. , Blood 2010; 116(18): 3418

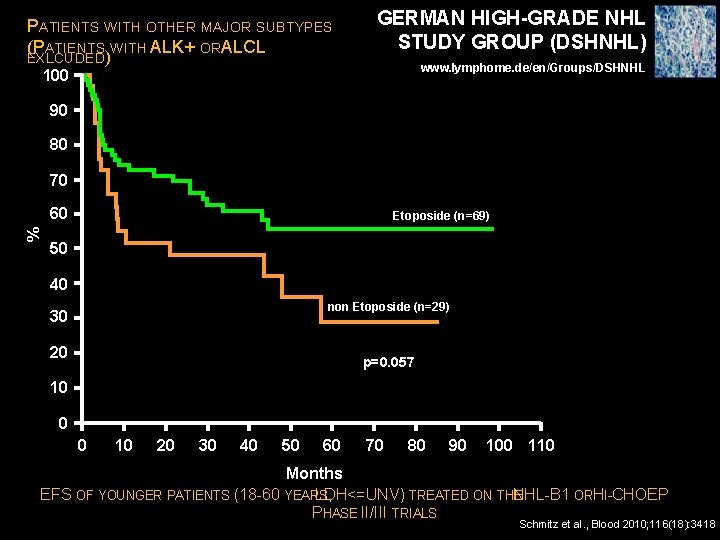
PATIENTS WITH OTHER MAJOR SUBTYPES (PATIENTS WITH ALK+ ORA LCL EXLCUDED) GERMAN HIGH-GRADE NHL STUDY GROUP (DSHNHL) www. lymphome. de/en/Groups/DSHNHL 100 90 80 70 % 60 Etoposide (n=69) 50 40 non Etoposide (n=29) 30 20 p=0. 057 10 0 0 10 20 30 40 50 60 70 80 90 100 110 Months EFS OF YOUNGER PATIENTS (18 -60 YEARS LDH<=UNV) , TREATED ON THE NHL-B 1 ORH I-CHOEP PHASE II/III TRIALS Schmitz et al. , Blood 2010; 116(18): 3418

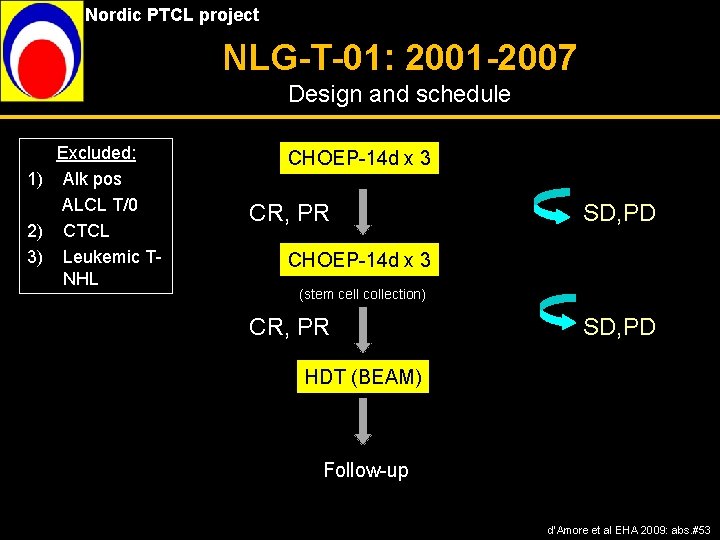
Nordic PTCL project NLG-T-01: 2001 -2007 Design and schedule Excluded: 1) Alk pos ALCL T/0 2) CTCL 3) Leukemic TNHL CHOEP-14 d x 3 CR, PR SD, PD CHOEP-14 d x 3 (stem cell collection) CR, PR SD, PD HDT (BEAM) Follow-up d’Amore et al EHA 2009: abs. #53

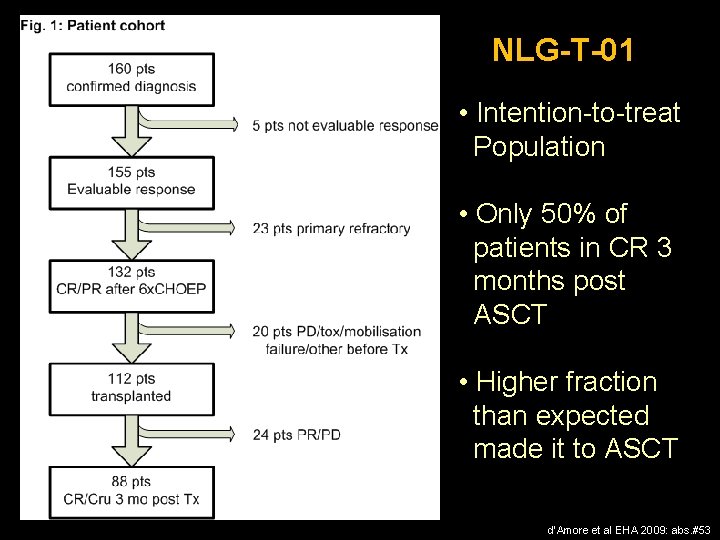
NLG-T-01 • Intention-to-treat Population • Only 50% of patients in CR 3 months post ASCT • Higher fraction than expected made it to ASCT d’Amore et al EHA 2009: abs. #53

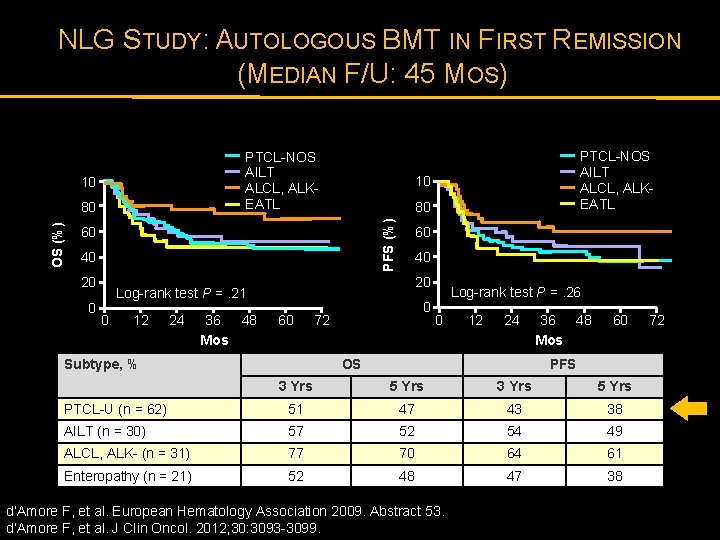
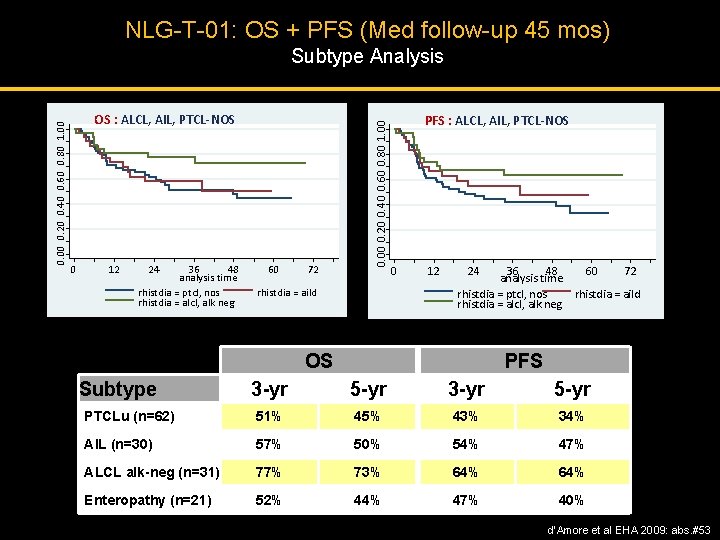
NLG STUDY: AUTOLOGOUS BMT IN FIRST REMISSION (MEDIAN F/U: 45 MOS) PTCL-NOS AILT ALCL, ALKEATL 10 10 80 PFS (%) OS (%) 80 60 40 20 Log-rank test P =. 21 0 12 24 36 48 Mos PTCL-NOS AILT ALCL, ALKEATL 60 Subtype, % 0 72 Log-rank test P =. 26 0 12 24 OS 36 48 Mos 60 PFS 3 Yrs 5 Yrs PTCL-U (n = 62) 51 47 43 38 AILT (n = 30) 57 52 54 49 ALCL, ALK- (n = 31) 77 70 64 61 Enteropathy (n = 21) 52 48 47 38 d’Amore F, et al. European Hematology Association 2009. Abstract 53. d’Amore F, et al. J Clin Oncol. 2012; 30: 3093 -3099. 72

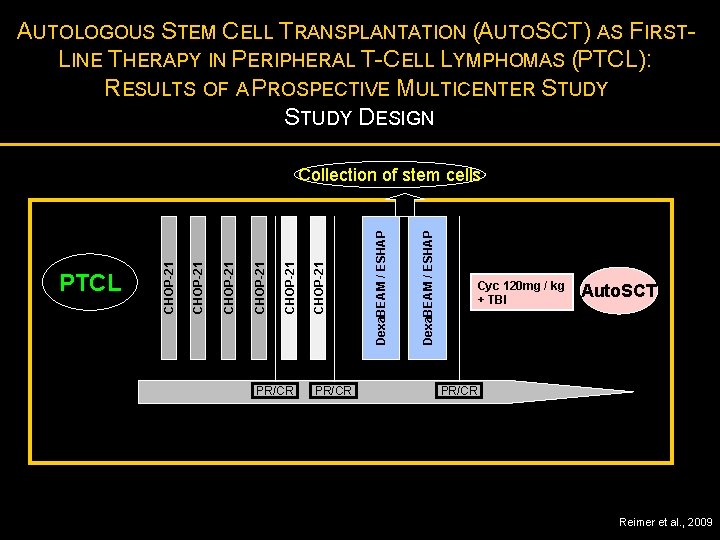
AUTOLOGOUS STEM CELL TRANSPLANTATION (AUTOSCT) AS FIRSTLINE THERAPY IN PERIPHERAL T-CELL LYMPHOMAS (PTCL): RESULTS OF A PROSPECTIVE MULTICENTER STUDY DESIGN PR/CR Dexa. BEAM / ESHAP CHOP-21 CHOP-21 PTCL CHOP-21 Collection of stem cells Cyc 120 mg / kg + TBI Auto. SCT PR/CR Reimer et al. , 2009

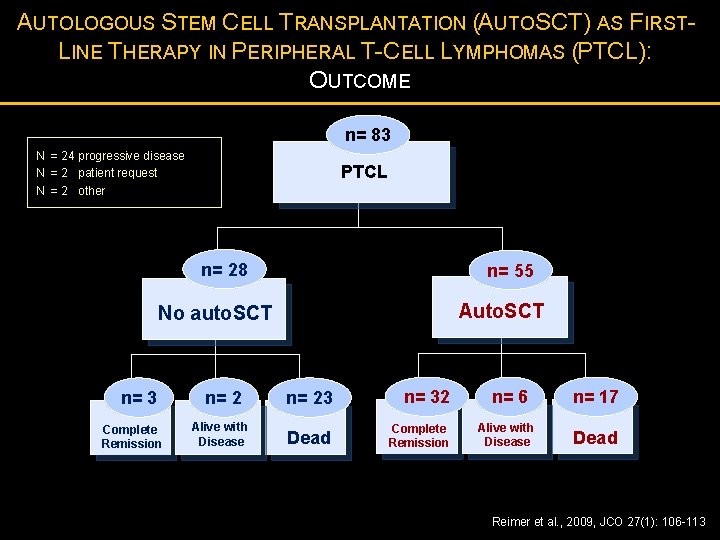
AUTOLOGOUS STEM CELL TRANSPLANTATION (AUTOSCT) AS FIRSTLINE THERAPY IN PERIPHERAL T-CELL LYMPHOMAS (PTCL): OUTCOME n= 83 N = 24 progressive disease N = 2 patient request N = 2 other PTCL n= 28 n= 55 Auto. SCT No auto. SCT n= 3 Complete Remission n= 2 Alive with Disease n= 23 n= 32 Dead Complete Remission n= 6 Alive with Disease n= 17 Dead Reimer et al. , 2009, JCO 27(1): 106 -113

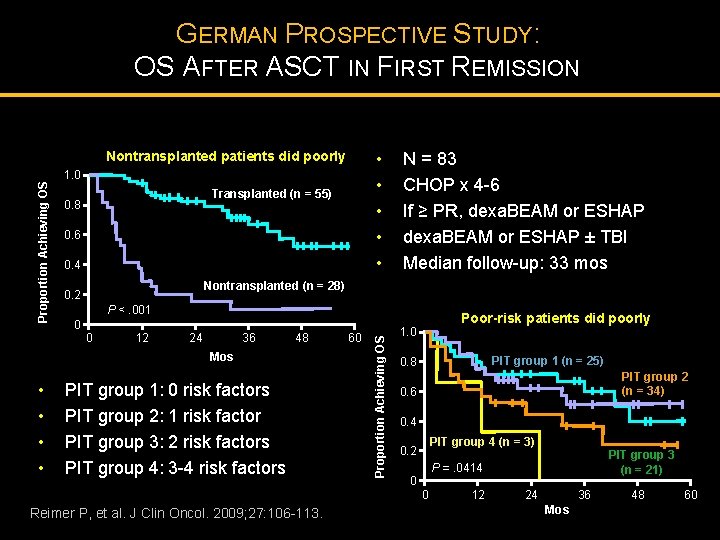
GERMAN PROSPECTIVE STUDY: OS AFTER ASCT IN FIRST REMISSION Transplanted (n = 55) 0. 8 0. 6 0. 4 N = 83 CHOP x 4 -6 If ≥ PR, dexa. BEAM or ESHAP ± TBI Median follow-up: 33 mos Nontransplanted (n = 28) 0. 2 P <. 001 0 0 12 24 36 48 Mos • • • 1. 0 PIT group 1: 0 risk factors PIT group 2: 1 risk factor PIT group 3: 2 risk factors PIT group 4: 3 -4 risk factors Reimer P, et al. J Clin Oncol. 2009; 27: 106 -113. 60 Proportion Achieving OS Nontransplanted patients did poorly Poor-risk patients did poorly 1. 0 PIT group 1 (n = 25) 0. 8 PIT group 2 (n = 34) 0. 6 0. 4 PIT group 4 (n = 3) 0. 2 0 PIT group 3 (n = 21) P =. 0414 0 12 24 36 Mos 48 60

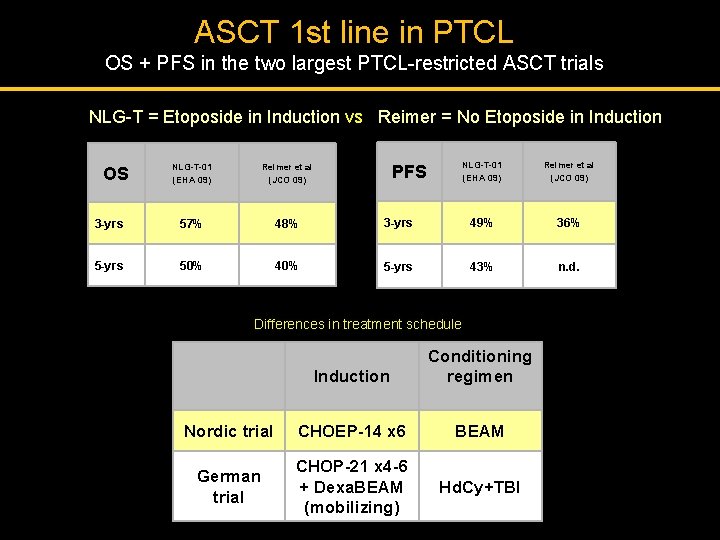
ASCT 1 st line in PTCL OS + PFS in the two largest PTCL-restricted ASCT trials NLG-T = Etoposide in Induction vs Reimer = No Etoposide in Induction NLG-T-01 (EHA 09) Reimer et al (JCO 09) 3 -yrs 57% 48% 5 -yrs 50% 40% OS NLG-T-01 (EHA 09) Reimer et al (JCO 09) 3 -yrs 49% 36% 5 -yrs 43% n. d. PFS Differences in treatment schedule Induction Conditioning regimen Nordic trial CHOEP-14 x 6 BEAM German trial CHOP-21 x 4 -6 + Dexa. BEAM (mobilizing) Hd. Cy+TBI

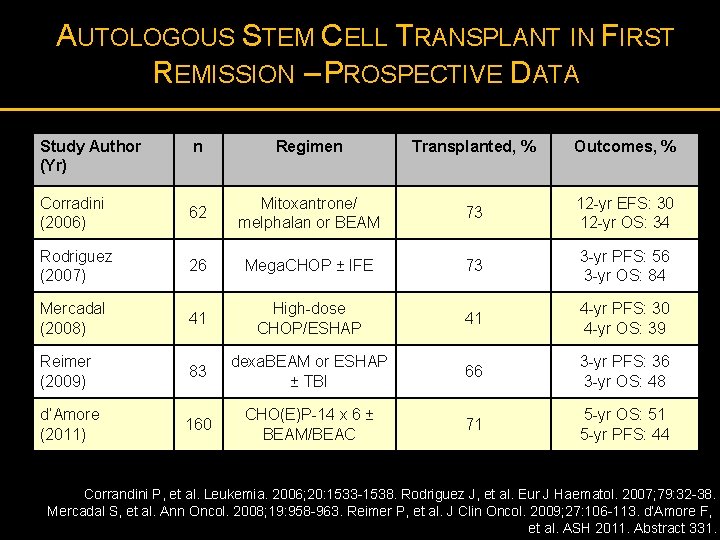
AUTOLOGOUS STEM CELL TRANSPLANT IN FIRST REMISSION – PROSPECTIVE DATA Study Author (Yr) n Regimen Transplanted, % Outcomes, % Corradini (2006) 62 Mitoxantrone/ melphalan or BEAM 73 12 -yr EFS: 30 12 -yr OS: 34 Rodriguez (2007) 26 Mega. CHOP ± IFE 73 3 -yr PFS: 56 3 -yr OS: 84 Mercadal (2008) 41 High-dose CHOP/ESHAP 41 4 -yr PFS: 30 4 -yr OS: 39 Reimer (2009) 83 dexa. BEAM or ESHAP ± TBI 66 3 -yr PFS: 36 3 -yr OS: 48 d’Amore (2011) 160 CHO(E)P-14 x 6 ± BEAM/BEAC 71 5 -yr OS: 51 5 -yr PFS: 44 Corrandini P, et al. Leukemia. 2006; 20: 1533 -1538. Rodriguez J, et al. Eur J Haematol. 2007; 79: 32 -38. Mercadal S, et al. Ann Oncol. 2008; 19: 958 -963. Reimer P, et al. J Clin Oncol. 2009; 27: 106 -113. d’Amore F, et al. ASH 2011. Abstract 331.

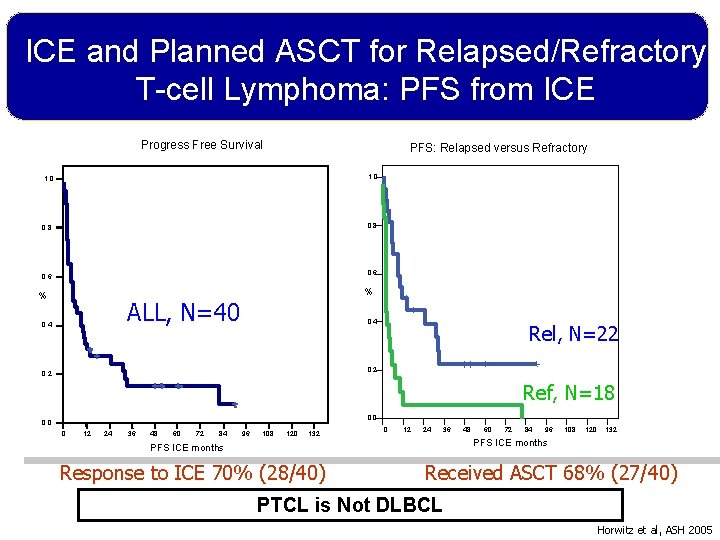
ICE and Planned ASCT for Relapsed/Refractory T-cell Lymphoma: PFS from ICE Progress Free Survival PFS: Relapsed versus Refractory 1. 0 0. 8 0. 6 % % ALL, N=40 0. 4 Rel, N=22 0. 2 Ref, N=18 0. 0 0 12 24 36 48 60 72 84 96 108 120 132 0 12 24 36 60 72 84 96 108 120 132 PFS ICE months Response to ICE 70% (28/40) 48 Received ASCT 68% (27/40) PTCL is Not DLBCL Horwitz et al, ASH 2005

PERIPHERAL T-CELL LYMPHOMAS: HOW I MANAGE FRONTLINE DISEASE · Putting The T-cell Lymphomas in Context · What Are the Optimal Upfront Treatment Considerations · Bridging Patients with Relapsed or Refractory Disease with Novel Drugs · Conclusion

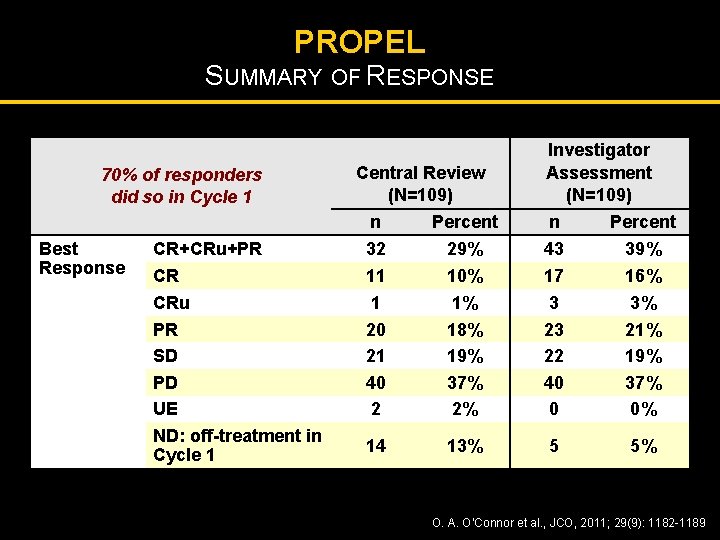
PROPEL SUMMARY OF RESPONSE 70% of responders did so in Cycle 1 Best Response Central Review (N=109) n Percent Investigator Assessment (N=109) n Percent CR+CRu+PR 32 29% 43 39% CR CRu PR SD PD UE ND: off-treatment in Cycle 1 11 1 20 21 40 2 10% 1% 18% 19% 37% 2% 17 3 23 22 40 0 16% 3% 21% 19% 37% 0% 14 13% 5 5% O. A. O’Connor et al. , JCO, 2011; 29(9): 1182 -1189

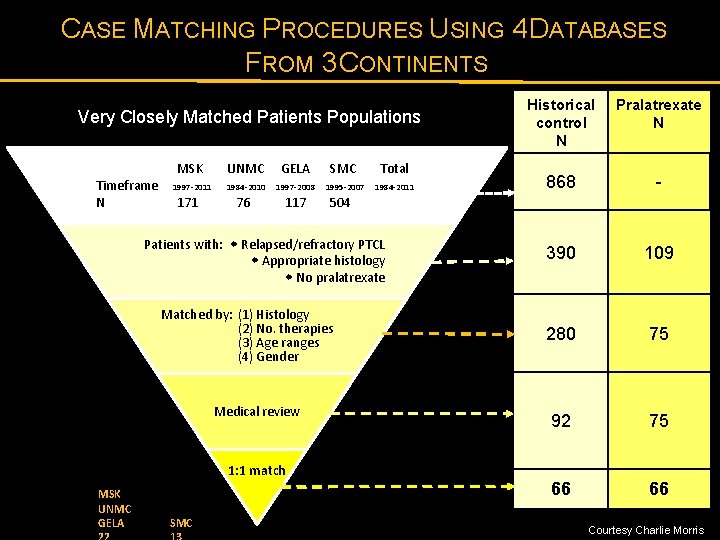
CASE MATCHING PROCEDURES USING 4 DATABASES FROM 3 CONTINENTS Very Closely Matched Patients Populations Timeframe N MSK UNMC GELA SMC Total 1997 -2011 1984 -2010 1997 -2008 1995 -2007 1984 -2011 171 76 117 504 Medical review 1: 1 match MSK UNMC GELA SMC Pralatrexate N 868 - 390 109 280 75 92 75 66 66 868 Patients with: Relapsed/refractory PTCL Appropriate histology No pralatrexate Matched by: (1) Histology (2) No. therapies (3) Age ranges (4) Gender Historical control N Courtesy Charlie Morris

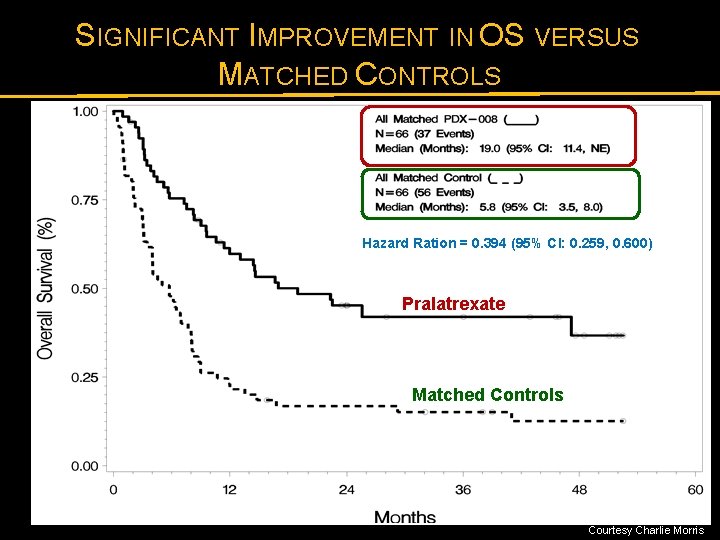
SIGNIFICANT IMPROVEMENT IN OS VERSUS MATCHED CONTROLS Hazard Ration = 0. 394 (95% CI: 0. 259, 0. 600) Pralatrexate Matched Controls Courtesy Charlie Morris

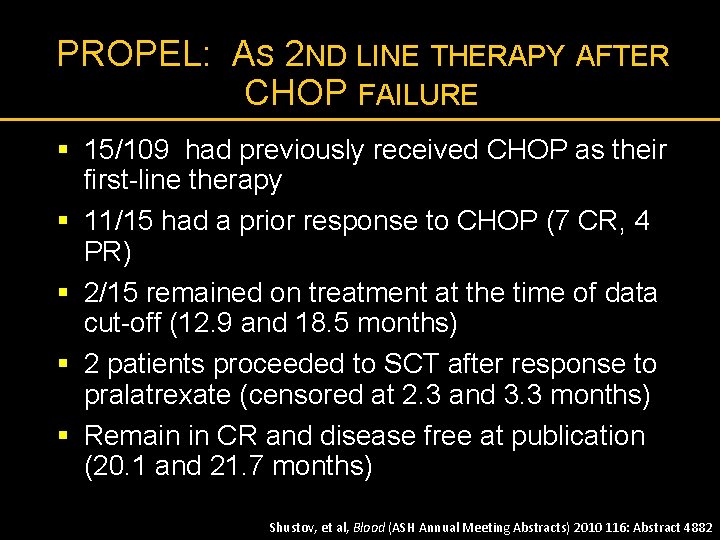
PROPEL: AS 2 ND LINE THERAPY AFTER CHOP FAILURE § 15/109 had previously received CHOP as their first-line therapy § 11/15 had a prior response to CHOP (7 CR, 4 PR) § 2/15 remained on treatment at the time of data cut-off (12. 9 and 18. 5 months) § 2 patients proceeded to SCT after response to pralatrexate (censored at 2. 3 and 3. 3 months) § Remain in CR and disease free at publication (20. 1 and 21. 7 months) Shustov, et al, Blood (ASH Annual Meeting Abstracts) 2010 116: Abstract 4882

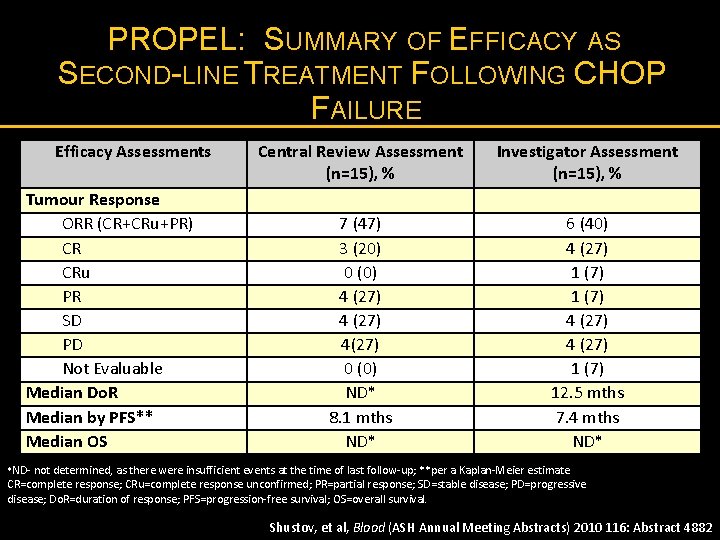
PROPEL: SUMMARY OF EFFICACY AS SECOND-LINE TREATMENT FOLLOWING CHOP FAILURE Efficacy Assessments Tumour Response ORR (CR+CRu+PR) CR CRu PR SD PD Not Evaluable Median Do. R Median by PFS** Median OS Central Review Assessment (n=15), % Investigator Assessment (n=15), % 7 (47) 3 (20) 0 (0) 4 (27) 4(27) 0 (0) ND* 8. 1 mths ND* 6 (40) 4 (27) 1 (7) 12. 5 mths 7. 4 mths ND* *ND- not determined, as there were insufficient events at the time of last follow-up; **per a Kaplan-Meier estimate CR=complete response; CRu=complete response unconfirmed; PR=partial response; SD=stable disease; PD=progressive disease; Do. R=duration of response; PFS=progression-free survival; OS=overall survival. Shustov, et al, Blood (ASH Annual Meeting Abstracts) 2010 116: Abstract 4882

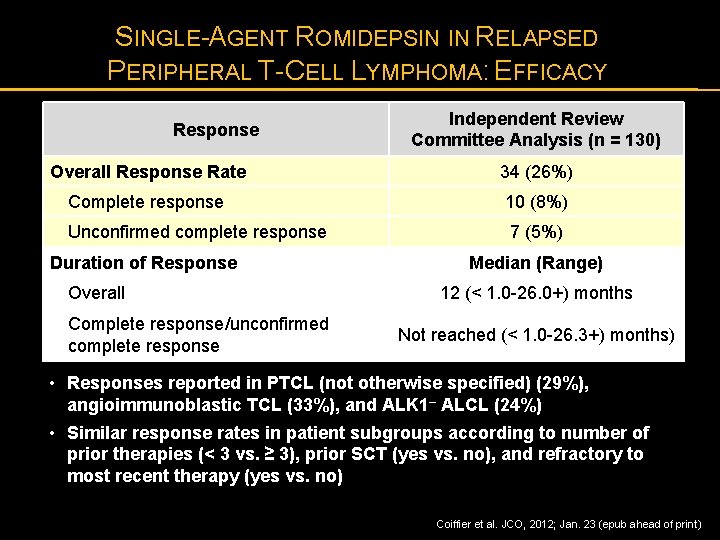
SINGLE-AGENT ROMIDEPSIN IN RELAPSED PERIPHERAL T-CELL LYMPHOMA: EFFICACY Response Independent Review Committee Analysis (n = 130) Overall Response Rate 34 (26%) Complete response 10 (8%) Unconfirmed complete response 7 (5%) Duration of Response Overall Complete response/unconfirmed complete response Median (Range) 12 (< 1. 0 -26. 0+) months Not reached (< 1. 0 -26. 3+) months) • Responses reported in PTCL (not otherwise specified) (29%), angioimmunoblastic TCL (33%), and ALK 1– ALCL (24%) • Similar response rates in patient subgroups according to number of prior therapies (< 3 vs. ≥ 3), prior SCT (yes vs. no), and refractory to most recent therapy (yes vs. no) Coiffier et al. JCO, 2012; Jan. 23 (epub ahead of print)

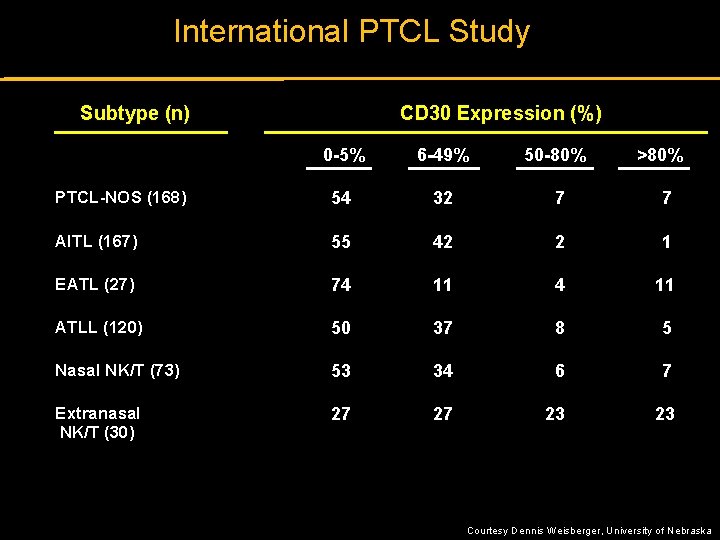
International PTCL Study Subtype (n) CD 30 Expression (%) 0 -5% 6 -49% 50 -80% >80% PTCL-NOS (168) 54 32 7 7 AITL (167) 55 42 2 1 EATL (27) 74 11 ATLL (120) 50 37 8 5 Nasal NK/T (73) 53 34 6 7 Extranasal NK/T (30) 27 27 23 23 Courtesy Dennis Weisberger, University of Nebraska

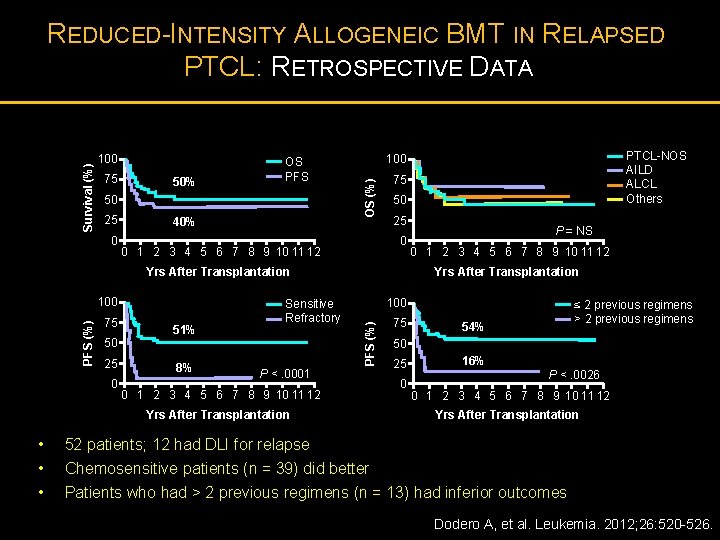
100 75 50% OS PFS 50 25 0 40% 75 50 25 0 0 1 2 3 4 5 6 7 8 9 10 11 12 Yrs After Transplantation 75 50 25 0 51% 8% Sensitive Refractory 0 1 2 3 4 5 6 7 8 9 10 11 12 100 P <. 0001 0 1 2 3 4 5 6 7 8 9 10 11 12 Yrs After Transplantation • • • P = NS Yrs After Transplantation PFS (%) 100 PTCL-NOS AILD ALCL Others 100 OS (%) Survival (%) REDUCED-INTENSITY ALLOGENEIC BMT IN RELAPSED PTCL: RETROSPECTIVE DATA 75 ≤ 2 previous regimens > 2 previous regimens 54% 50 25 0 16% P <. 0026 0 1 2 3 4 5 6 7 8 9 10 11 12 Yrs After Transplantation 52 patients; 12 had DLI for relapse Chemosensitive patients (n = 39) did better Patients who had > 2 previous regimens (n = 13) had inferior outcomes Dodero A, et al. Leukemia. 2012; 26: 520 -526.

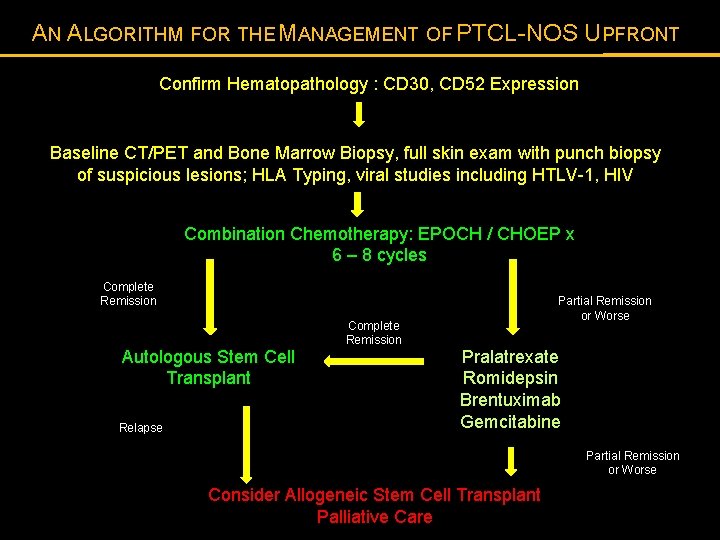
AN ALGORITHM FOR THE MANAGEMENT OF PTCL-NOS UPFRONT Confirm Hematopathology : CD 30, CD 52 Expression Baseline CT/PET and Bone Marrow Biopsy, full skin exam with punch biopsy of suspicious lesions; HLA Typing, viral studies including HTLV-1, HIV Combination Chemotherapy: EPOCH / CHOEP x 6 – 8 cycles Complete Remission Autologous Stem Cell Transplant Relapse Complete Remission Partial Remission or Worse Pralatrexate Romidepsin Brentuximab Gemcitabine Partial Remission or Worse Consider Allogeneic Stem Cell Transplant Palliative Care

Peripheral T-Cell Lymphomas: How I Manage Frontline Disease • CHOP clearly inferior, but is a regulatory standard. Integrating etoposide may provide advantage? • Caution removal of doxorubicin based upon the T-cell Lymphoma Project • Consolidation with ASCT probably beneficial, and more so then in the relapsed or refractory setting • Allogeneic stem cell transplant should be considered in eligible patients with R/R disease • USE NEWER FDA APPROVED DRUGS earlier in the natural history of the disease. • Many new studies emerging as CHOP additions (Pralatrexate, Romidepsin, Brentuximab vedotin, Belinostat)

THANK YOU

Peripheral T-Cell Lymphomas: How I Manage Frontline Disease · Putting The T-cell Lymphomas in Context · Defining ‘Standards of Care’ for Upfront Treatment (won’t take long) · Newly Emerging Options for Advanced (R/R) Disease: Is combination the right way to go? - Pralatrexate in Relapsed/Refractory PTCL - Targeting the Epigenome in PTCL - Targeting CD 30+ T-cell Malignancies · Conclusion

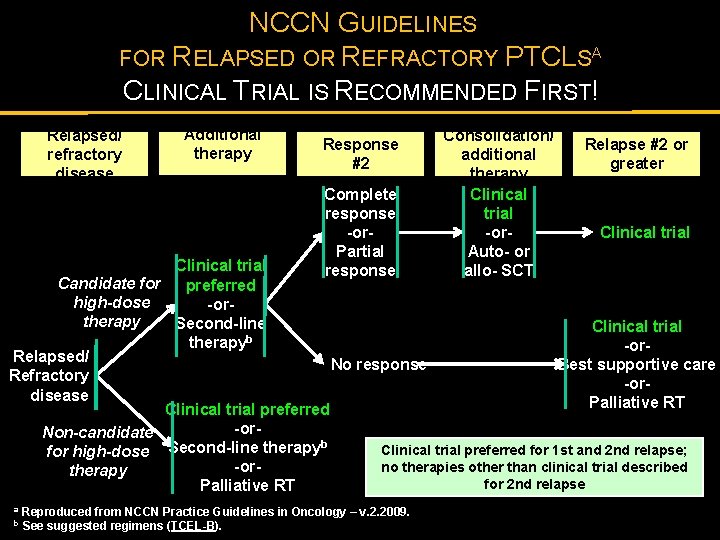
NCCN GUIDELINES FOR RELAPSED OR REFRACTORY PTCLSA CLINICAL TRIAL IS RECOMMENDED FIRST! Relapsed/ refractory disease Additional therapy Response #2 Complete response -or. Partial response Consolidation/ additional therapy Clinical trial -or. Auto- or allo- SCT Relapse #2 or greater Clinical trial Candidate for preferred high-dose -ortherapy Second-line Clinical trial b therapy -or. Relapsed/ Best supportive care No response Refractory -ordisease Palliative RT Clinical trial preferred -or. Non-candidate b Clinical trial preferred for 1 st and 2 nd relapse; for high-dose Second-line therapy no therapies other than clinical trial described -ortherapy for 2 nd relapse Palliative RT a b Reproduced from NCCN Practice Guidelines in Oncology – v. 2. 2009. See suggested regimens (TCEL-B).

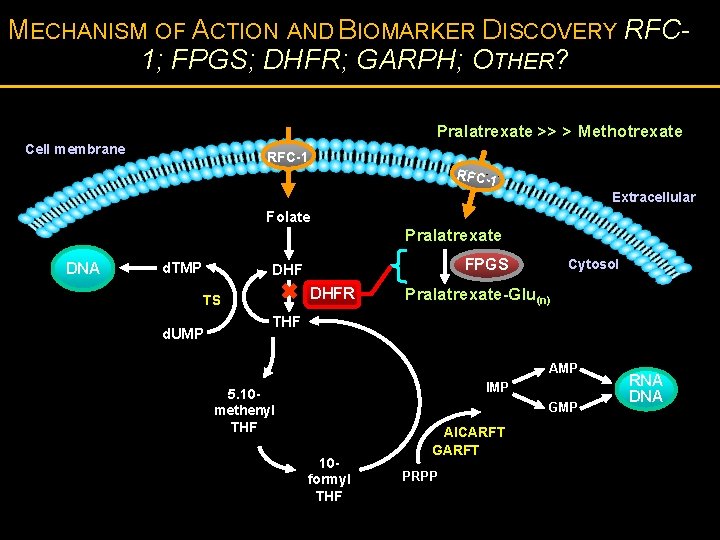
MECHANISM OF ACTION AND BIOMARKER DISCOVERY RFC 1; FPGS; DHFR; GARPH; OTHER? Pralatrexate >> > Methotrexate Cell membrane RFC-1 Extracellular Folate Pralatrexate DNA d. TMP FPGS DHFR TS d. UMP Cytosol Pralatrexate-Glu(n) THF AMP IMP 5. 10 methenyl THF GMP 10 formyl THF AICARFT GARFT PRPP RNA DNA

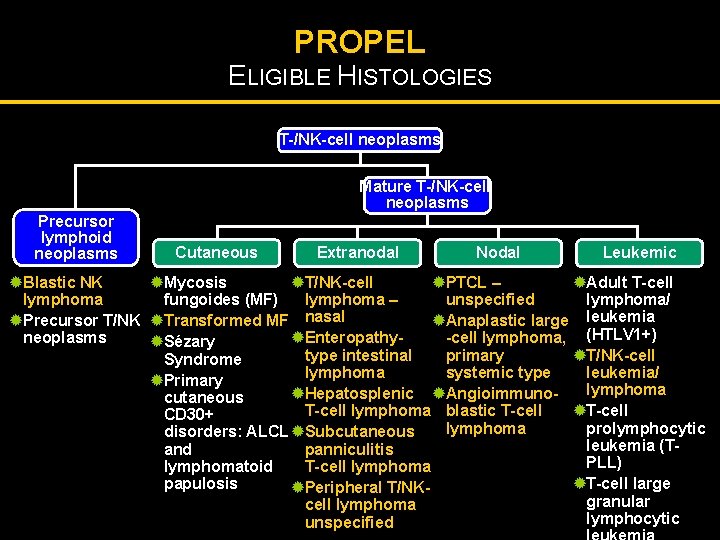
PROPEL ELIGIBLE HISTOLOGIES T-/NK-cell neoplasms Mature T-/NK-cell neoplasms Precursor lymphoid neoplasms Cutaneous Extranodal Nodal Leukemic ®Blastic NK ®Mycosis ®T/NK-cell ®PTCL – ®Adult T-cell lymphoma fungoides (MF) lymphoma – unspecified lymphoma/ ®Precursor T/NK ®Transformed MF nasal ®Anaplastic large leukemia ®Enteropathyneoplasms -cell lymphoma, (HTLV 1+) ®Sézary type intestinal ®T/NK-cell primary Syndrome lymphoma leukemia/ systemic type ®Primary lymphoma ®Hepatosplenic ®Angioimmunocutaneous T-cell lymphoma blastic T-cell ®T-cell CD 30+ prolymphocytic lymphoma disorders: ALCL ®Subcutaneous leukemia (Tpanniculitis and PLL) T-cell lymphomatoid ®T-cell large papulosis ®Peripheral T/NKgranular cell lymphoma lymphocytic unspecified Adapted from Jaffe et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 2001.

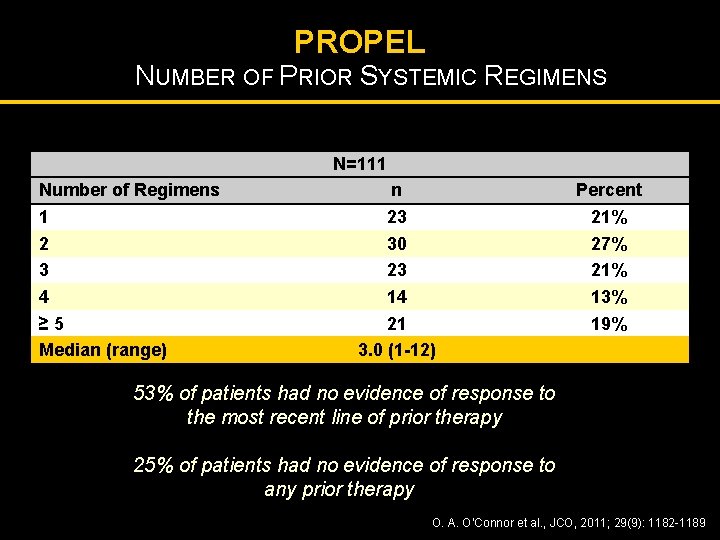
PROPEL NUMBER OF PRIOR SYSTEMIC REGIMENS N=111 Number of Regimens 1 2 3 4 ≥ 5 Median (range) n 23 30 23 14 21 3. 0 (1 -12) Percent 21% 27% 21% 13% 19% 53% of patients had no evidence of response to the most recent line of prior therapy 25% of patients had no evidence of response to any prior therapy O. A. O’Connor et al. , JCO, 2011; 29(9): 1182 -1189

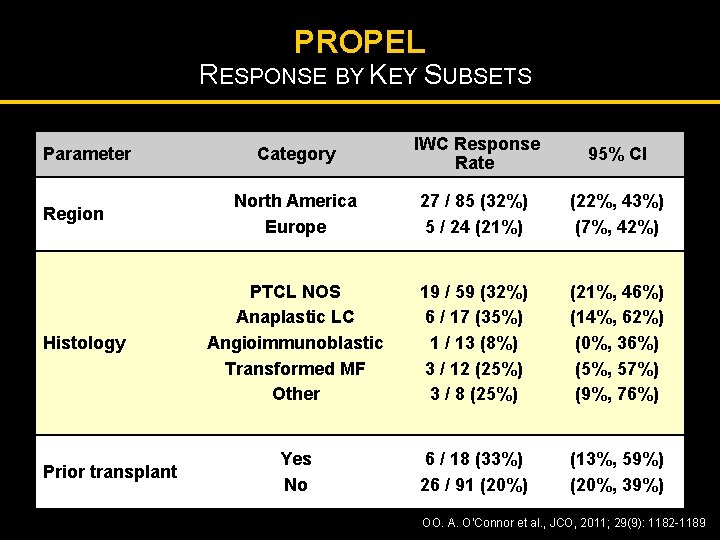
PROPEL RESPONSE BY KEY SUBSETS Parameter Region Histology Prior transplant Category IWC Response Rate 95% CI North America Europe 27 / 85 (32%) 5 / 24 (21%) (22%, 43%) (7%, 42%) PTCL NOS Anaplastic LC Angioimmunoblastic Transformed MF Other 19 / 59 (32%) 6 / 17 (35%) 1 / 13 (8%) 3 / 12 (25%) 3 / 8 (25%) (21%, 46%) (14%, 62%) (0%, 36%) (5%, 57%) (9%, 76%) Yes No 6 / 18 (33%) 26 / 91 (20%) (13%, 59%) (20%, 39%) OO. A. O’Connor et al. , JCO, 2011; 29(9): 1182 -1189

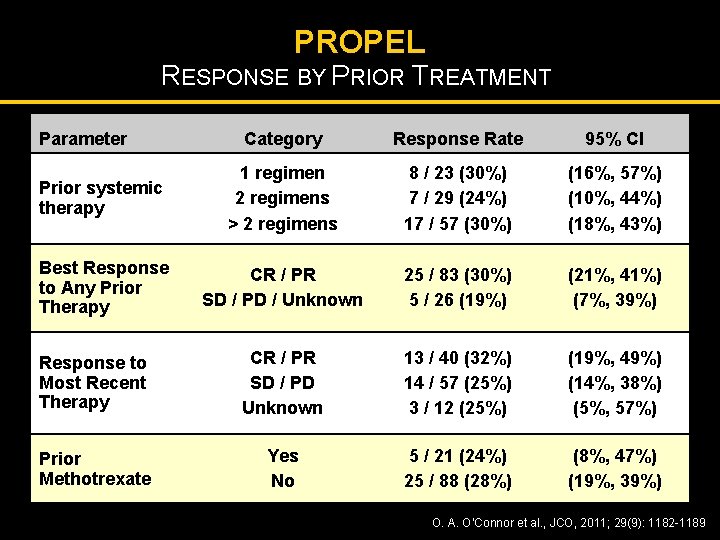
PROPEL RESPONSE BY PRIOR TREATMENT Parameter Category Response Rate 95% CI Prior systemic therapy 1 regimen 2 regimens > 2 regimens 8 / 23 (30%) 7 / 29 (24%) 17 / 57 (30%) (16%, 57%) (10%, 44%) (18%, 43%) Best Response to Any Prior Therapy CR / PR SD / PD / Unknown 25 / 83 (30%) 5 / 26 (19%) (21%, 41%) (7%, 39%) Response to Most Recent Therapy CR / PR SD / PD Unknown 13 / 40 (32%) 14 / 57 (25%) 3 / 12 (25%) (19%, 49%) (14%, 38%) (5%, 57%) Prior Methotrexate Yes No 5 / 21 (24%) 25 / 88 (28%) (8%, 47%) (19%, 39%) O. A. O’Connor et al. , JCO, 2011; 29(9): 1182 -1189

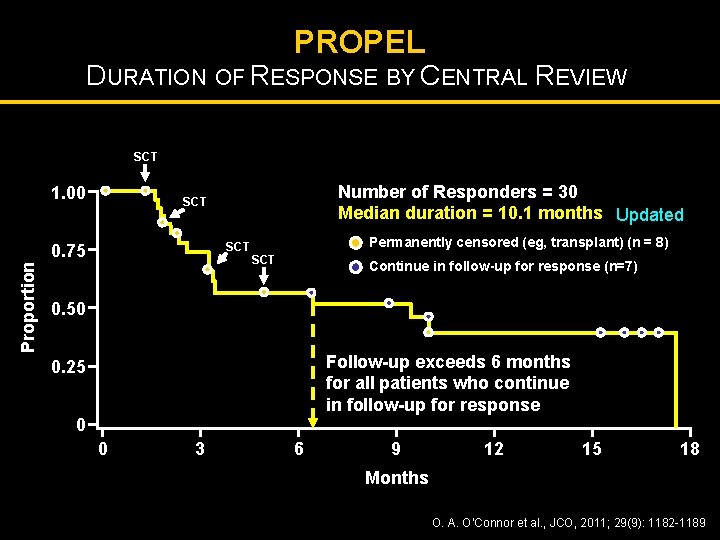
PROPEL DURATION OF RESPONSE BY CENTRAL REVIEW SCT 1. 00 SCT 0. 75 Proportion Number of Responders = 30 Median duration = 10. 1 months Updated SCT Permanently censored (eg, transplant) (n = 8) SCT Continue in follow-up for response (n=7) 0. 50 Follow-up exceeds 6 months for all patients who continue in follow-up for response 0. 25 0 0 3 6 9 12 15 18 Months O. A. O’Connor et al. , JCO, 2011; 29(9): 1182 -1189

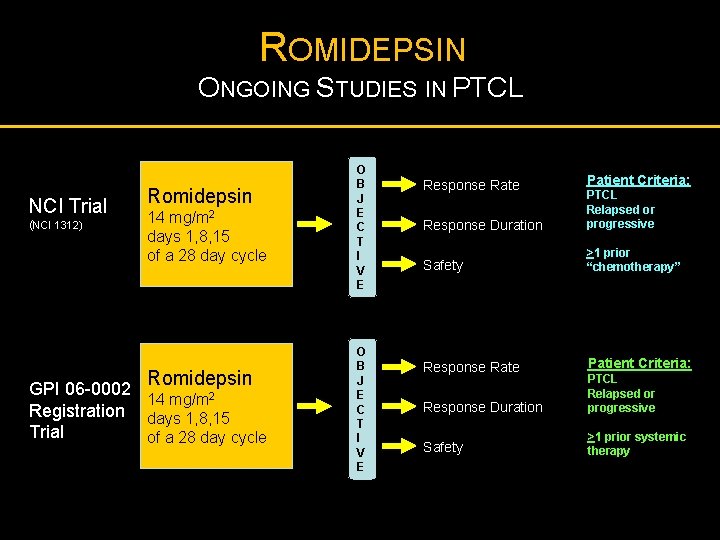
ROMIDEPSIN ONGOING STUDIES IN PTCL NCI Trial (NCI 1312) Romidepsin 14 mg/m 2 days 1, 8, 15 of a 28 day cycle Romidepsin GPI 06 -0002 14 mg/m 2 Registration days 1, 8, 15 Trial of a 28 day cycle O B J E C T I V E Response Rate Patient Criteria: Response Duration PTCL Relapsed or progressive Safety >1 prior “chemotherapy” Response Rate Patient Criteria: Response Duration PTCL Relapsed or progressive Safety >1 prior systemic therapy

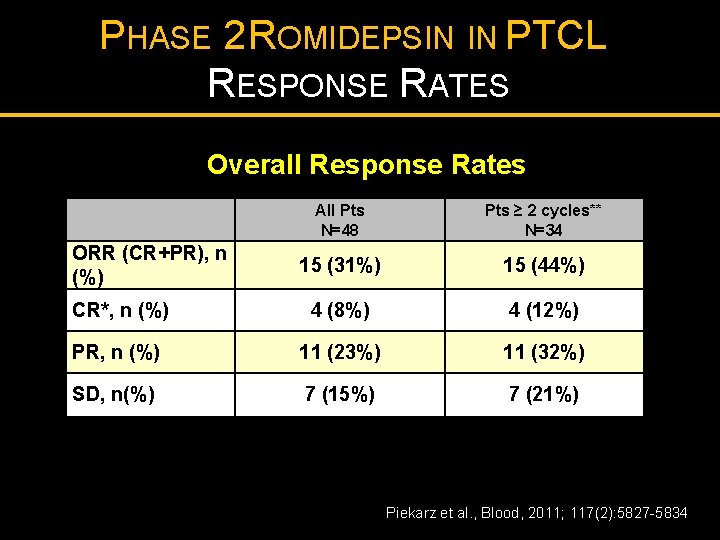
PHASE 2 ROMIDEPSIN IN PTCL RESPONSE RATES Overall Response Rates All Pts N=48 Pts ≥ 2 cycles** N=34 15 (31%) 15 (44%) CR*, n (%) 4 (8%) 4 (12%) PR, n (%) 11 (23%) 11 (32%) SD, n(%) 7 (15%) 7 (21%) ORR (CR+PR), n (%) s Piekarz et al. , Blood, 2011; 117(2): 5827 -5834

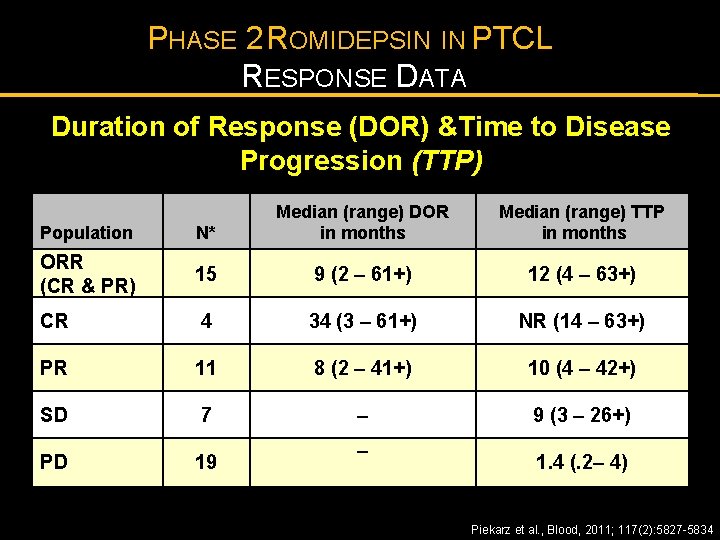
PHASE 2 ROMIDEPSIN IN PTCL RESPONSE DATA Duration of Response (DOR) &Time to Disease Progression (TTP) Median time to first response was 1. 8 (1 -6) months Population N* Median (range) DOR in months ORR (CR & PR) 15 9 (2 – 61+) 12 (4 – 63+) CR 4 34 (3 – 61+) NR (14 – 63+) PR 11 8 (2 – 41+) 10 (4 – 42+) SD 7 – 9 (3 – 26+) PD 19 – Median (range) TTP in months 1. 4 (. 2– 4) *7 patients were not evaluable; NR, median not yet reached; + denotes continuing response Piekarz et al. , Blood, 2011; 117(2): 5827 -5834

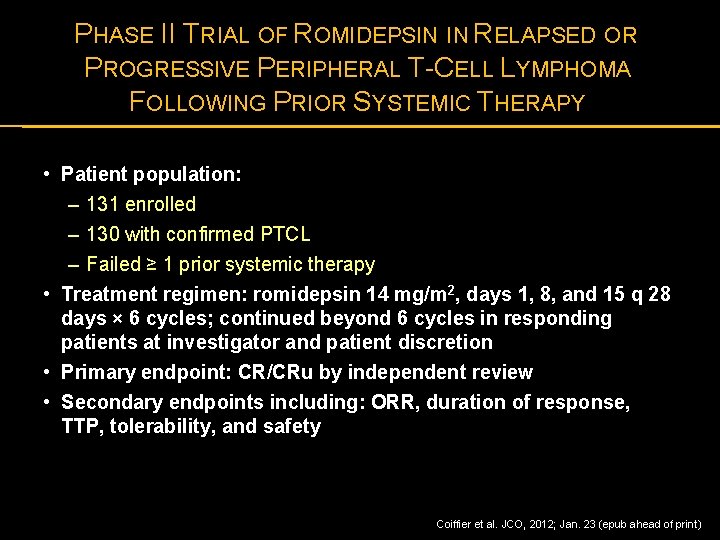
PHASE II TRIAL OF ROMIDEPSIN IN RELAPSED OR PROGRESSIVE PERIPHERAL T-CELL LYMPHOMA FOLLOWING PRIOR SYSTEMIC THERAPY • Patient population: – 131 enrolled – 130 with confirmed PTCL – Failed ≥ 1 prior systemic therapy • Treatment regimen: romidepsin 14 mg/m 2, days 1, 8, and 15 q 28 days × 6 cycles; continued beyond 6 cycles in responding patients at investigator and patient discretion • Primary endpoint: CR/CRu by independent review • Secondary endpoints including: ORR, duration of response, TTP, tolerability, and safety Coiffier et al. JCO, 2012; Jan. 23 (epub ahead of print)

Peripheral T-Cell Lymphomas: How I Manage Frontline Disease · Putting The T-cell Lymphomas in Context · Defining ‘Standards of Care’ for Upfront Treatment (won’t take long) · Newly Emerging Options for Advanced (R/R) Disease: Is combination the right way to go? - Pralatrexate in Relapsed/Refractory PTCL - Targeting the Epigenome in PTCL - Targeting CD 30+ T-cell Malignancies · Conclusion

CD 30 Expression in in Non-Lymphoma Tumors § § Acute Myeloid Leukemia/Granulocytic Sarcoma § – M Fickers 1996, J Clin Pathol 49: 7623; R Horie 1999, Am J of Path. 155: 2029 -41 § ALL and AML – Gattei 1997, Blood 89: 2048 -59 § § Mastocytosis § § M Garcia-Prats 1998, Histopathology 32: 462 -472 Sotlar 2010, Modern Pathology, 1 -11 Mesothelioma – M Garcia-Prats 1998, Histopathology 32: 462 -472; C Dunphy 2000, Arch Pathol Lab Med 124: 1077 -79 Nasopharyngeal carcinoma § § Kneile 2006, Histopathology 48: 855861 Testicular embryonal carcinoma – J Ferreiro 1994, Hum. Pathol. 25: 522524; K Iczkowski 2008, Hum. Pathol. 39: 275 -281 § Lung (Adenocarcinoma) § § – Internal data on file Bone sarcomas – G Mechtersheimer 1990, Cancer 66: 1732 -37 Melanoma Thyroid Carcinoma (Medullary, Papillary) – M Trovato 2001, Thyroid 11: 621 -8 § Cervical, Head and Neck SCC (Viralassociated cancers), Lung, Breast, Endometrial, Ovarian, Prostate, & Melanoma – Human protein atlas (http: //www. proteinatlas. org/ENSG 0000 0120949/cancer)

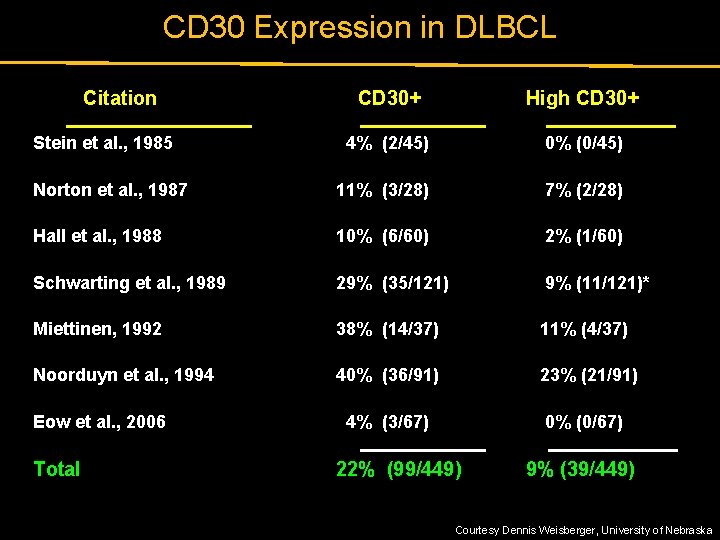
CD 30 Expression in DLBCL Citation CD 30+ High CD 30+ 4% (2/45) 0% (0/45) Norton et al. , 1987 11% (3/28) 7% (2/28) Hall et al. , 1988 10% (6/60) 2% (1/60) Schwarting et al. , 1989 29% (35/121) 9% (11/121)* Miettinen, 1992 38% (14/37) 11% (4/37) Noorduyn et al. , 1994 40% (36/91) 23% (21/91) 4% (3/67) 0% (0/67) Stein et al. , 1985 Eow et al. , 2006 Total 22% (99/449) 9% (39/449) Courtesy Dennis Weisberger, University of Nebraska

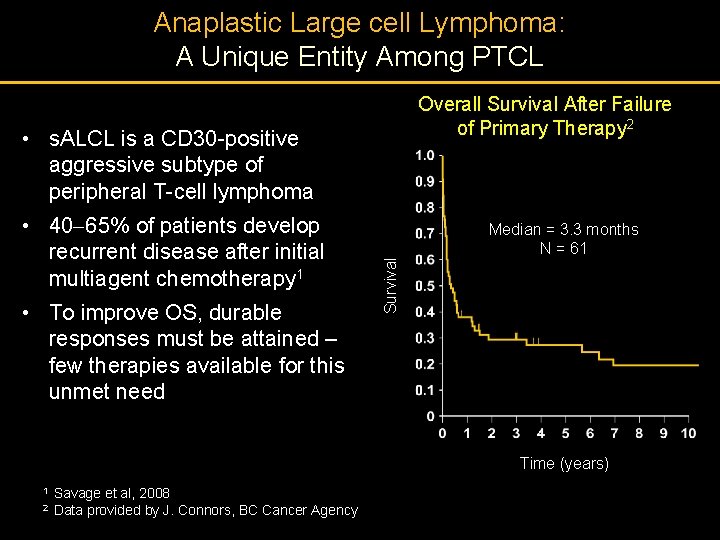
Anaplastic Large cell Lymphoma: A Unique Entity Among PTCL Overall Survival After Failure of Primary Therapy 2 • 40 65% of patients develop recurrent disease after initial multiagent chemotherapy 1 • To improve OS, durable responses must be attained – few therapies available for this unmet need Survival • s. ALCL is a CD 30 -positive aggressive subtype of peripheral T-cell lymphoma Median = 3. 3 months N = 61 Time (years) 1 Savage et al, 2008 2 Data provided by J. Connors, BC Cancer Agency

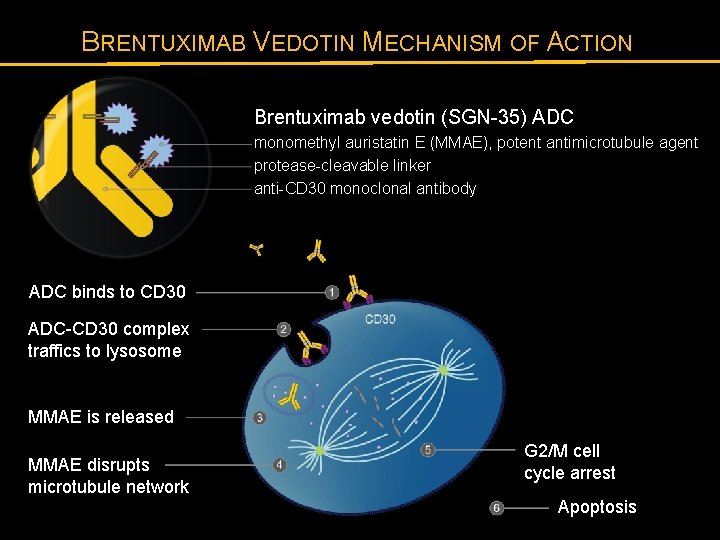
BRENTUXIMAB VEDOTIN MECHANISM OF ACTION Brentuximab vedotin (SGN-35) ADC monomethyl auristatin E (MMAE), potent antimicrotubule agent protease-cleavable linker anti-CD 30 monoclonal antibody ADC binds to CD 30 ADC-CD 30 complex traffics to lysosome MMAE is released MMAE disrupts microtubule network G 2/M cell cycle arrest Apoptosis

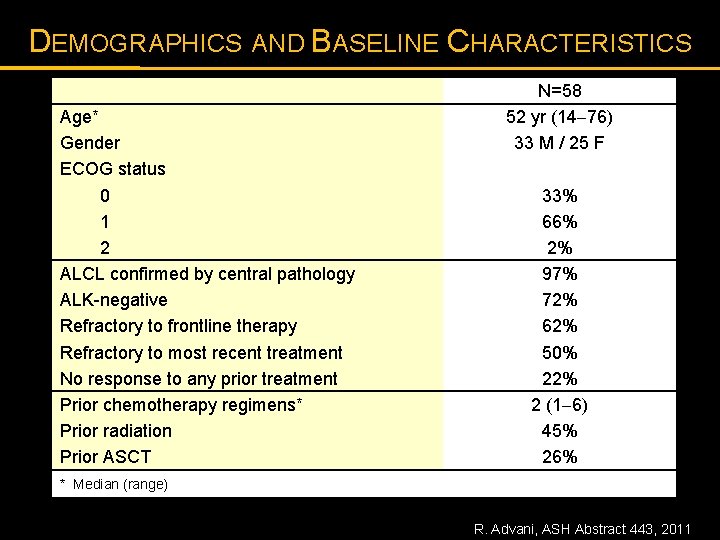
DEMOGRAPHICS AND BASELINE CHARACTERISTICS Age* Gender ECOG status 0 1 2 ALCL confirmed by central pathology ALK-negative Refractory to frontline therapy Refractory to most recent treatment No response to any prior treatment Prior chemotherapy regimens* Prior radiation Prior ASCT N=58 52 yr (14 76) 33 M / 25 F 33% 66% 2% 97% 72% 62% 50% 22% 2 (1 6) 45% 26% * Median (range) R. Advani, ASH Abstract 443, 2011

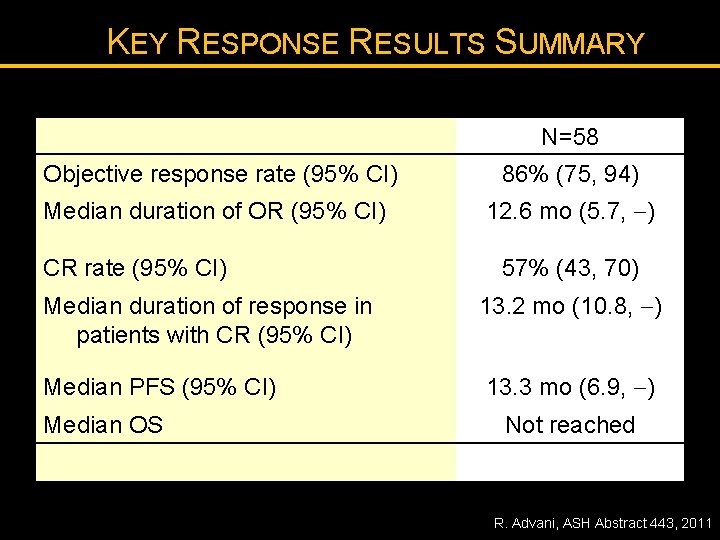
KEY RESPONSE RESULTS SUMMARY N=58 Objective response rate (95% CI) 86% (75, 94) Median duration of OR (95% CI) 12. 6 mo (5. 7, ) CR rate (95% CI) 57% (43, 70) Median duration of response in patients with CR (95% CI) 13. 2 mo (10. 8, ) Median PFS (95% CI) 13. 3 mo (6. 9, ) Median OS Not reached R. Advani, ASH Abstract 443, 2011

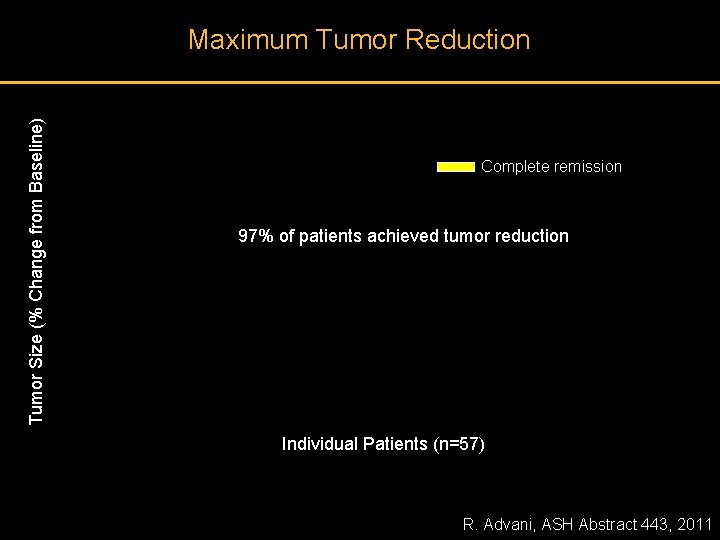
Tumor Size (% Change from Baseline) Maximum Tumor Reduction Complete remission 97% of patients achieved tumor reduction Individual Patients (n=57) R. Advani, ASH Abstract 443, 2011

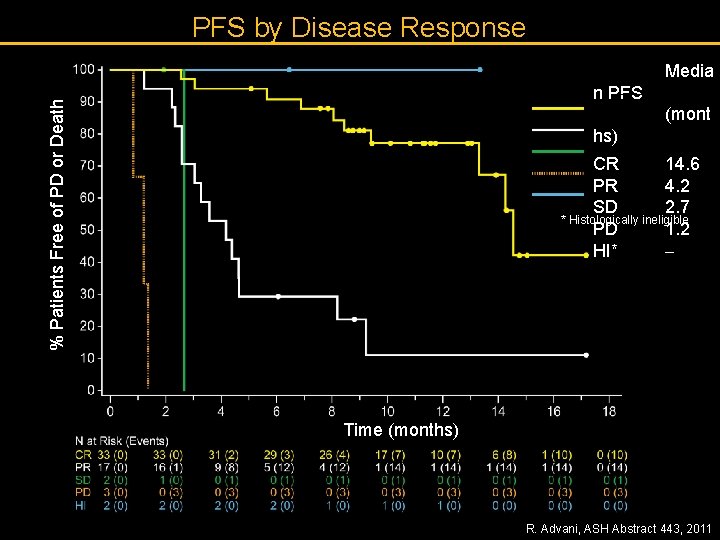
PFS by Disease Response Media % Patients Free of PD or Death n PFS (mont hs) CR 14. 6 PR 4. 2 SD 2. 7 * Histologically ineligible PD 1. 2 HI* Time (months) R. Advani, ASH Abstract 443, 2011

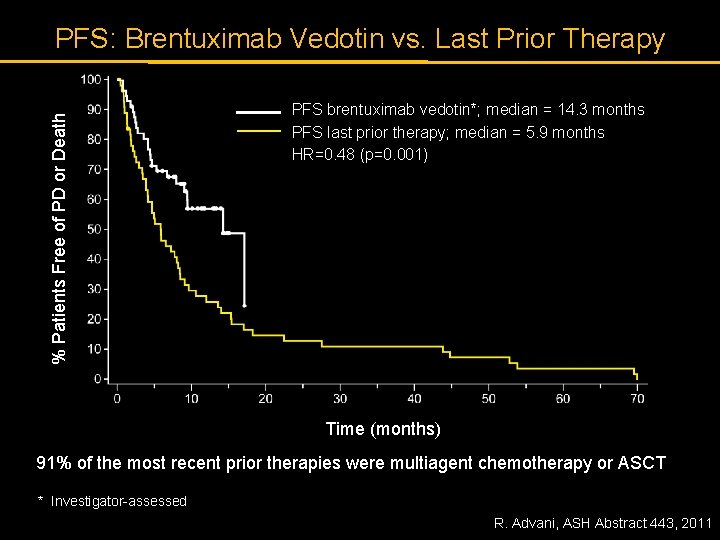
% Patients Free of PD or Death PFS: Brentuximab Vedotin vs. Last Prior Therapy PFS brentuximab vedotin*; median = 14. 3 months PFS last prior therapy; median = 5. 9 months HR=0. 48 (p=0. 001) Time (months) 91% of the most recent prior therapies were multiagent chemotherapy or ASCT * Investigator-assessed R. Advani, ASH Abstract 443, 2011

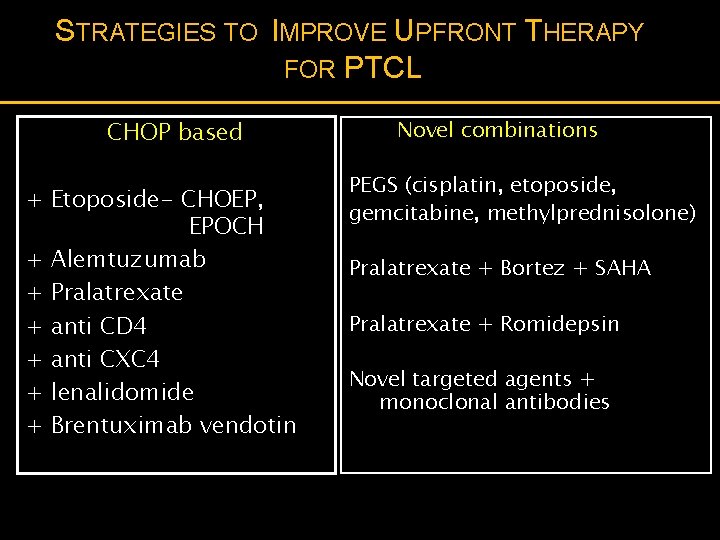
STRATEGIES TO IMPROVE UPFRONT THERAPY FOR PTCL CHOP based + Etoposide- CHOEP, EPOCH + Alemtuzumab + Pralatrexate + anti CD 4 + anti CXC 4 + lenalidomide + Brentuximab vendotin Novel combinations PEGS (cisplatin, etoposide, gemcitabine, methylprednisolone) Pralatrexate + Bortez + SAHA Pralatrexate + Romidepsin Novel targeted agents + monoclonal antibodies

Thank You

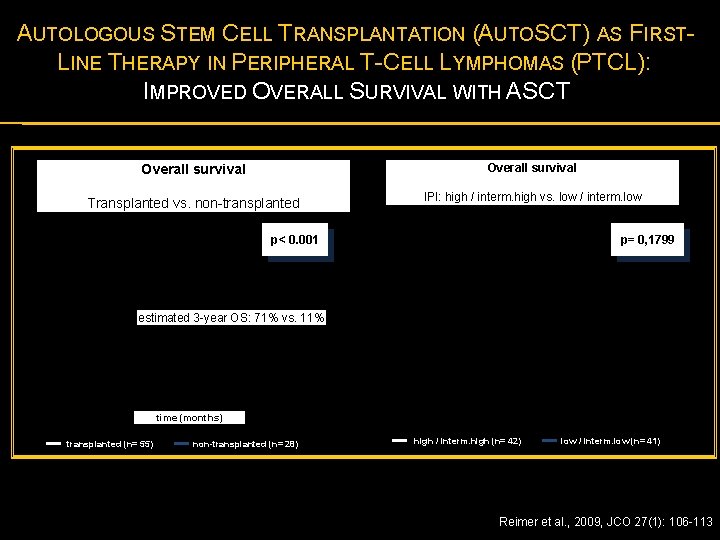
AUTOLOGOUS STEM CELL TRANSPLANTATION (AUTOSCT) AS FIRSTLINE THERAPY IN PERIPHERAL T-CELL LYMPHOMAS (PTCL): IMPROVED OVERALL SURVIVAL WITH ASCT Overall survival Transplanted vs. non-transplanted IPI: high / interm. high vs. low / interm. low p< 0. 001 p= 0, 1799 estimated 3 -year OS: 71% vs. 11% time (months) transplanted (n= 55) non-transplanted (n= 28) high / interm. high (n= 42) low / interm. low (n= 41) Reimer et al. , 2009, JCO 27(1): 106 -113

NLG-T-01: OS + PFS (Med follow-up 45 mos) OS : ALCL, AIL, PTCL-NOS 0 12 24 36 48 analysis time rhistdia = ptcl, nos rhistdia = alcl, alk neg 60 72 0. 00 0. 20 0. 40 0. 60 0. 80 1. 00 Subtype Analysis rhistdia = aild PFS : ALCL, AIL, PTCL-NOS 0 12 24 36 48 60 72 analysis time rhistdia = ptcl, nos rhistdia = aild rhistdia = alcl, alk neg OS Subtype PFS 3 -yr 5 -yr PTCLu (n=62) 51% 45% 43% 34% AIL (n=30) 57% 50% 54% 47% ALCL alk-neg (n=31) 77% 73% 64% Enteropathy (n=21) 52% 44% 47% 40% d’Amore et al EHA 2009: abs. #53

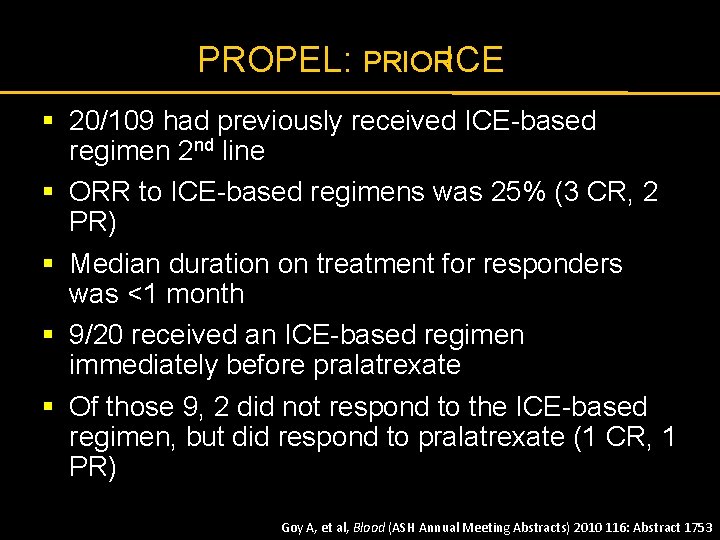
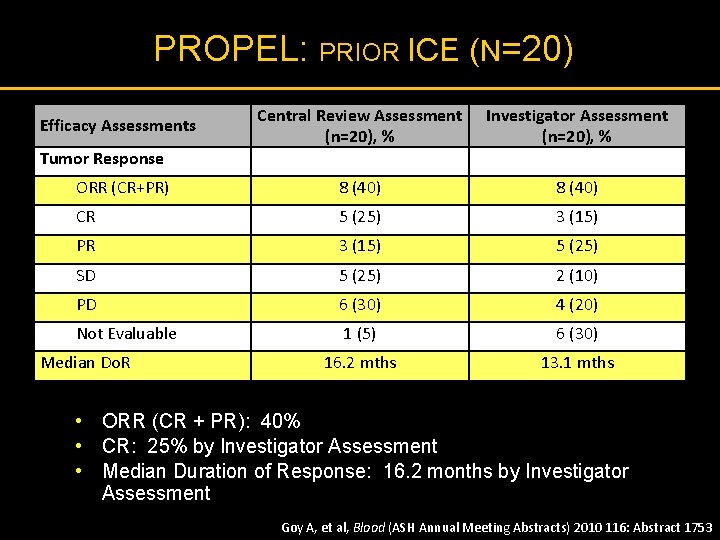
PROPEL: PRIORICE § 20/109 had previously received ICE-based regimen 2 nd line § ORR to ICE-based regimens was 25% (3 CR, 2 PR) § Median duration on treatment for responders was <1 month § 9/20 received an ICE-based regimen immediately before pralatrexate § Of those 9, 2 did not respond to the ICE-based regimen, but did respond to pralatrexate (1 CR, 1 PR) Goy A, et al, Blood (ASH Annual Meeting Abstracts) 2010 116: Abstract 1753

PROPEL: PRIOR ICE (N=20) Central Review Assessment (n=20), % Investigator Assessment (n=20), % ORR (CR+PR) 8 (40) CR 5 (25) 3 (15) PR 3 (15) 5 (25) SD 5 (25) 2 (10) PD 6 (30) 4 (20) Not Evaluable 1 (5) 6 (30) 16. 2 mths 13. 1 mths Efficacy Assessments Tumor Response Median Do. R • ORR (CR + PR): 40% • CR: 25% by Investigator Assessment • Median Duration of Response: 16. 2 months by Investigator Assessment Goy A, et al, Blood (ASH Annual Meeting Abstracts) 2010 116: Abstract 1753