ISOIEC 17025 2017 Whats new IAEA SSDL and
















- Slides: 19

ISO/IEC 17025: 2017 – What’s new IAEA – SSDL and Quality Management Training December 20, 2018 Stephen M. KEOCHAKIAN

New structure ISO/IEC 17025: 2005 1. Scope 2. Normative references 3. Terms and definitions 4. Management requirements 5. Technical requirements Annex A – Nominal crossreferences to ISO 9001: 2000 Annex B – Guidelines for establishing applications for specific fields ISO/IEC 17025: 2017 1. Scope 2. Normative references 3. Terms and definitions 4. General requirements 5. Structural requirements 6. Resource requirements 7. Process requirements 8. Management requirements Annex A – Metrological traceability Annex B – Management system 2

Why revise 17025? Align structure and content with other recently revised ISO standards To give laboratories more flexibility Update language to reflect current practices and technologies 3

What is different Different structure Ø But many requirements are unchanged New: You must manage your “risks” New flexibility: Ø “What to do and not how to do it” is no longer the intention Ø Disappeared: “quality manual”, “quality manager”, “subcontracting”, etc. ) ØYou can still use them! This talk will cover some of the major changes 4

New structure ISO/IEC 17025: 2005 1. Scope 2. Normative references 3. Terms and definitions 4. Management requirements 5. Technical requirements Annex A – Nominal crossreferences to ISO 9001: 2000 Annex B – Guidelines for establishing applications for specific fields ISO/IEC 17025: 2017 1. Scope 2. Normative references 3. Terms and definitions 4. General requirements 5. Structural requirements 6. Resource requirements 7. Process requirements 8. Management requirements Annex A – Metrological traceability Annex B – Management system 5

New terms 6

New terms 3. 6 Laboratory “Laboratory”/“Laboratory activities” used throughout the document and refers to testing, calibration and sampling… 7

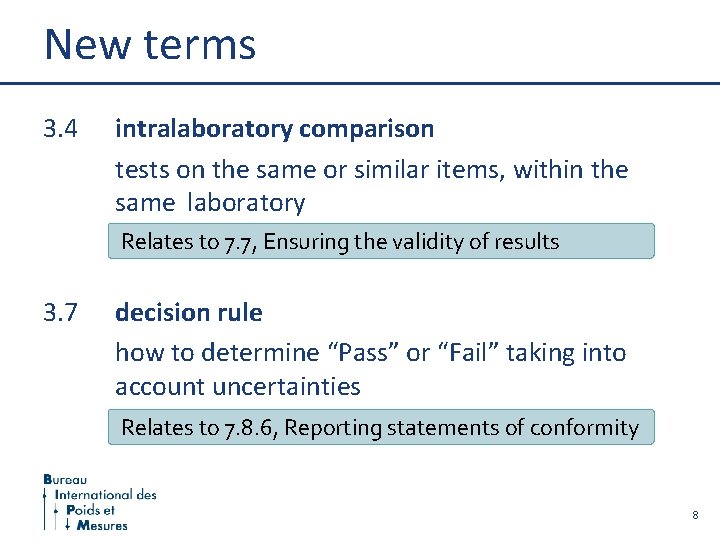
New terms 3. 4 intralaboratory comparison tests on the same or similar items, within the same laboratory Relates to 7. 7, Ensuring the validity of results 3. 7 decision rule how to determine “Pass” or “Fail” taking into account uncertainties Relates to 7. 8. 6, Reporting statements of conformity 8

New items 9

Risk & Opportunities NEW Risks and opportunities associated with the laboratory activities must be considered in order to: give assurance the management system achieves its intended results; enhance opportunities to achieve the purpose and objectives of the laboratory; prevent or reduce negative impacts and potential failures in the laboratory activities; achieves improvement. What does this mean for you? 10

4 Impartiality NEW 4. 1. 4 The laboratory shall … On an ongoing basis, the laboratory must identify risks to impartiality, including those arising from its activities or relationships or the relationships of its personnel. What does this mean for you? 11

4 Confidentiality Major Ø Ø Stronger emphasis on confidentiality New clauses n o i t c Ø Laboratories are to advise customers of the e t o information they will make publically ravailable. P Ø Are you publishing reports and nota telling the customer? t a D 12

6. 2 Your Staff Your staff (either internal or external) must be “SQEP” Suitably Qualified and Experienced Person This was already found in the 1705: 2005 version New: Emphasis is now on staff’s ability to not only identify departures from procedures, but also to evaluate the significance of these. New: Staff must be authorized to validate a procedure, etc. as well as following a procedure New: Competence must be monitored continuously 13

6. 2 Monitoring staff competence How do you do this? • • • Witnessed measurements Results from participation in intra-laboratory comparisons Results from participation in inter-laboratory comparisons Measuring test samples/instruments Findings from internal audits 14

Traceability 15

6. 5 Metrological traceability 16

Questions? 17

Helpful links: UKAS Assessor tutorial: https: //www. ukas. com/download/general_docume nts/UKAS-January-2018 -training-v 9. pdf NATA Gap Analysis template: https: //www. nata. com. au/phocadownload/genaccreditation-criteria/17025 -2017 -Gap-analysis. pdf UKAS Transition template: https: //www. ukas. com/download/publications/pu blications-relating-to-laboratory-accreditation/ISOIEC-17025 -2017 -Transition-Template. docx 18

Stephen Keochakian Bureau International Poids et Mesures Quality, Health, Safety, Environment Sèvres, France Office: +33 1 45076258 Mobile: +33 7 89440933 stephen. keochakian@bipm. org www. bipm. org
 Isoiec
Isoiec Daftar pertanyaan audit internal iso 17025:2017
Daftar pertanyaan audit internal iso 17025:2017 Facilities and environmental conditions
Facilities and environmental conditions Generalidades de la norma iso 9000
Generalidades de la norma iso 9000 Iso dumont
Iso dumont Iso 17025 laboratory management system
Iso 17025 laboratory management system Ntp 17025
Ntp 17025 Ntp 17025
Ntp 17025 Ntp iso 17025
Ntp iso 17025 55 miller street
55 miller street Ssr-5
Ssr-5 Saris iaea
Saris iaea Livechart iaea
Livechart iaea Succequent
Succequent Iaea gsr part 4
Iaea gsr part 4 Rtc protection film
Rtc protection film Iaea
Iaea Iaea
Iaea Stefano monti iaea
Stefano monti iaea Iaea
Iaea

































