UNI CEI EN ISOIEC 17025 ACCREDITATION AND ASSESSMENT



















- Slides: 41

UNI CEI EN ISO/IEC 17025 ACCREDITATION AND ASSESSMENT FOR PROBABILISTIC GENOTYPING: A MULTISOFTWARE STRATEGY FOR MIXTURE INTERPRETATON IN HIGHY CHALLENGING SAMPLES PAOLO GAROFANO; MONICA OMEDEI; GIUSEPPINA D’AMICO ; DENISE CANEPARO; VERONICA MORRA; SELENA CISANA, EUGENIO ALLADIO. HUMAN IDENTIFICATION SOLUTIONS (HIDS) – ROME 3 -4 MAY 2018

VERIFICATION PROVISION OF OBJECTIVE EVIDENCE THAT A GIVEN ITEM FULFILS SPECIFIED REQUIREMENTS (EG: CONFIRMATION THAT A TARGET MEASUREMENT UNCERTAINTY CAN BE MET) 17025: 2017 UNI CEI EN ISO/IEC 17025: 2005 VALIDATION THE GENERAL REQUIREMENTS FORGENERAL THE THISSPECIFIES INTERNATIONAL STANDARD SPECIFIES THE VERIFICATION , WHERE THEAND SPECIFIED COMPETENCE, IMPARTIALITY CONSISTENT REQUIREMENTS FOR THE COMPETENCE TO CARRY OUT OPERATION OFINCLUDING LABS. TESTS AND/OR CALIBRATIONS, SAMPLING. REQUIREMENTS ARE ADEQUATE FOR AN INTENDED USE DECISION RULE THAT DESCRIBES HOW MEASUREMENT UNCERTAINTY IS ACCOUNTED FOR WHEN STATING CONFORMITY WITH A SPECIFIED REQUIREMENT Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

ISO 17025 TECHNICAL REQUIREMENTS 5. 1 GENERAL 5. 2 PERSONNEL 5. 3 ACCOMMODATION AND ENVIRONMENTAL CONDITIONS 5. 4 TEST METHODS AND METHOD VALIDATION 5. 5 EQUIPMENT 5. 6 MEASUREMENT TRACEABILITY 5. 7 SAMPLING 5. 8 HANDLING OF ITEMS 5. 9 ASSURING THE QUALITY OF TESTS RESULTS 5. 10 REPORTING RESULTS Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

MIXTURE INTERPRETATION AND QAS NO LAB IN ITALY ACCREDITED FOR MIXTURE INTERPRETATION FEW LAB ALL OVER THE WORLD ACCRDITED UNDER QAS TRANSITION FROM CPI/CPE TO PROBABILISTIC GENOTYPING Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)


QAS AND MIXTURE INTERPRETATION NO LAB IN ITALY ACCREDITED FOR MIXTURE INTERPRETATION FEW LAB ALLOVER THE WORLD ACCRDITED UNDER QAS TRANSITION FROM CPI/CPE TO PROBABILISTIC GENOTYPING SCARCE GUIDELINES OR RECCOMANDATIONS FEW PROBABILISTIC SOFTWARE AVAILABLE INSUFFICIENT BIBLIOGRAPHY Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

CASEWORK EVIDENCE PROFILES 100 90 80 70 60 LT-DNA profile Total amplification 50 Mixture Single profile 40 30 20 10 0 2014 2015 2016 2017 Overall Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

ISO 17025 ACCREDITATION WORKFLOW CHOOSE WHAT IS THE LAB METHOD FOR EVALUATE METHODS’ VARIBILITIES AND REQUIREMENTS METHOD PROJECT MANAGMENT PERFORM VALIDATION STUDIES CHECK RESULTS THROUGH DATA ANALISYS ESTABLISH LABORATORY LIMITS METHOD ACCREDITATION Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

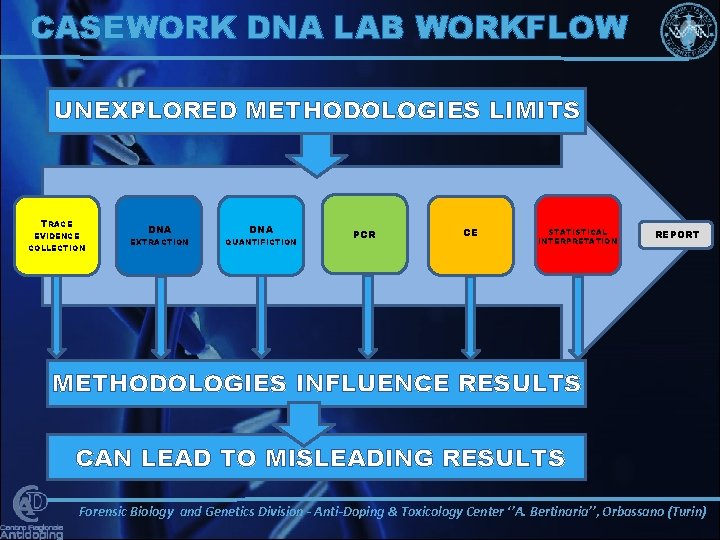
CASEWORK DNA LAB WORKFLOW UNEXPLORED METHODOLOGIES LIMITS TRACE EVIDENCE COLLECTION DNA EXTRACTION QUANTIFICTION PCR CE STATISTICAL INTERPRETATION REPORT METHODOLOGIES INFLUENCE RESULTS CAN LEAD TO MISLEADING RESULTS Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

PRE PCR UNCERTAINTY CONTRIBUTIONS CALIBRATION CURVE SX/Y CALIBRATION QUANTITATION (CALIBRATION CURVE) CALIBRATION ALIQUOTATION AUTOMATED DNA EXTRACTION 0. 0032 CURVE 0. 1003 CURVESX 0 0. 0003 REPEATABILITY RELATIVE SAMPLE /B REPEATABILITY 0. 0033 0. 0006 PIPETTE 100 UL 0. 0037 SCALE 50 REPEATABILITY OVERALL MG 0. 0008 ORAL SWAB 0. 0187 WHOLE BLOOD 0. 0005 UNCERTAINTY 15% Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

PCR INTERNAL VALIDATION SENSITIVITY REPEATIBILITY CONCORDANCE SPECIFICITY DEGRADATION INHIBITION MIXTURES/ MIXTURES DEGRADATION LT DNA MIXTURE PCR CYCLE VARIATION PCR ANNEALING TEMPERATURE VARIATION PCR DENATURATION TEMPERATURE VARIATION PCR EXTENSION TEMPERATURE VARIATION CE INJECTION TIME VARIATION CE VOLTAGE VARIATION Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

TYPING KITS VALIDATION STUDIES GLOBALFILER PCR AMPLIFICATION KIT® AMPFLSTR® NGM SELECT™ PCR AMPLIFICATION KIT AMPFLSTR® MINIFILER™ PCR AMPLIFICATION KIT AMPFLSTR® YFILER® PCR AMPLIFICATION KIT POWERPLEX® FUSION/FUSION 6 C SYSTEM POWERPLEX® ESI 17 FAST SYSTEM POWERPLEX® ESX 17 FAST SYSTEM POWERPLEX® Y 23 SYSTEM INVESIGATOR 24 PLEXQS KIT Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

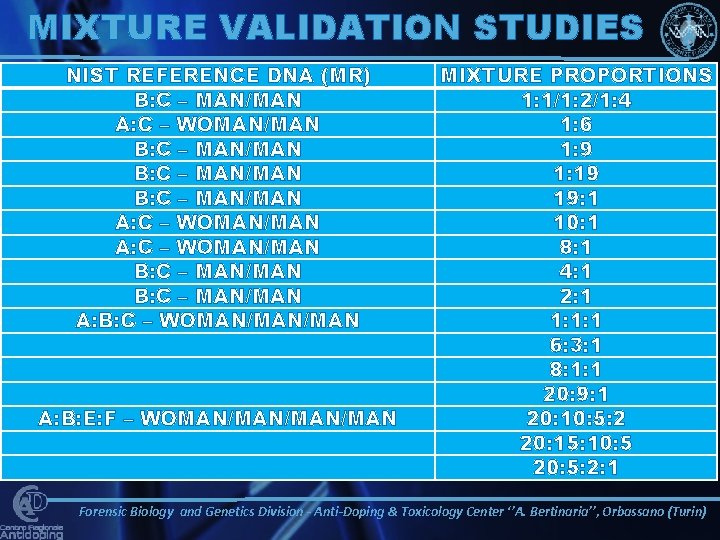
MIXTURE VALIDATION STUDIES NIST REFERENCE DNA (MR) B: C – MAN/MAN A: C – WOMAN/MAN B: C – MAN/MAN A: B: C – WOMAN/MAN A: B: E: F – WOMAN/MAN/MAN MIXTURE PROPORTIONS 1: 1/1: 2/1: 4 1: 6 1: 9 1: 19 19: 1 10: 1 8: 1 4: 1 2: 1 1: 1: 1 6: 3: 1 8: 1: 1 20: 9: 1 20: 10: 5: 2 20: 15: 10: 5 20: 5: 2: 1 Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

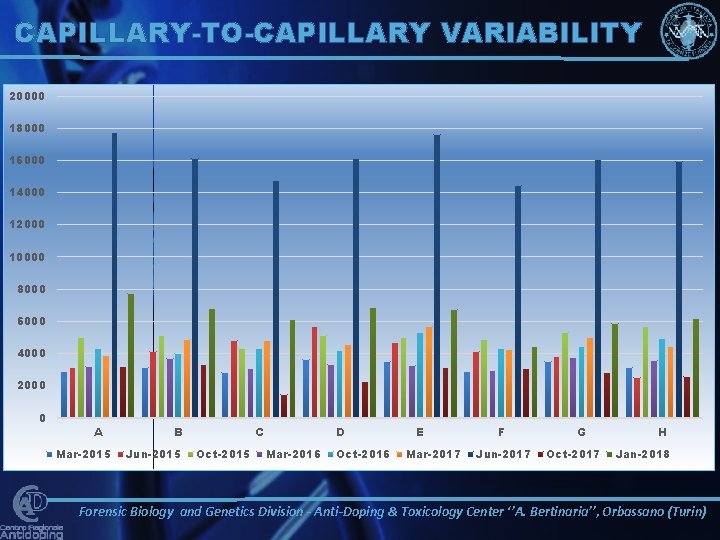
CAPILLARY-TO-CAPILLARY VARIABILITY 20000 18000 16000 14000 12000 10000 8000 6000 4000 2000 0 A Mar-2015 B Jun-2015 C Oct-2015 D Mar-2016 Oct-2016 E Mar-2017 F Jun-2017 G Oct-2017 H Jan-2018 Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

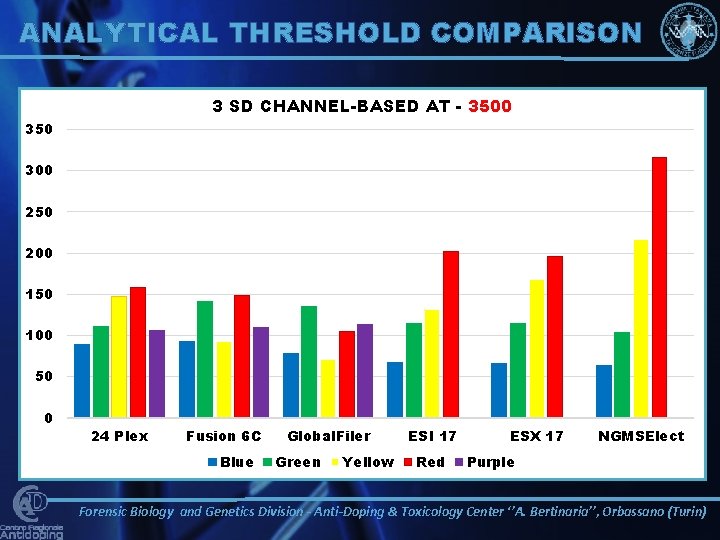
ANALYTICAL THRESHOLD COMPARISON 3 SD CHANNEL-BASED AT - 3500 350 300 250 200 150 100 50 0 24 Plex Fusion 6 C Blue Global. Filer Green Yellow ESI 17 Red ESX 17 NGMSElect Purple Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

ANALYTICAL THRESHOLD COMPARISON Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

MODELLING VARIABILITIES STUTTER MODELLING STOCHASTIC MODELLING DROP IN MODELLING DROP OUT MODELLING NOISE BACKGROUND MODELLING AT MODELLING PERFORMANCE CHECKS HAVE TO BE DONE Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

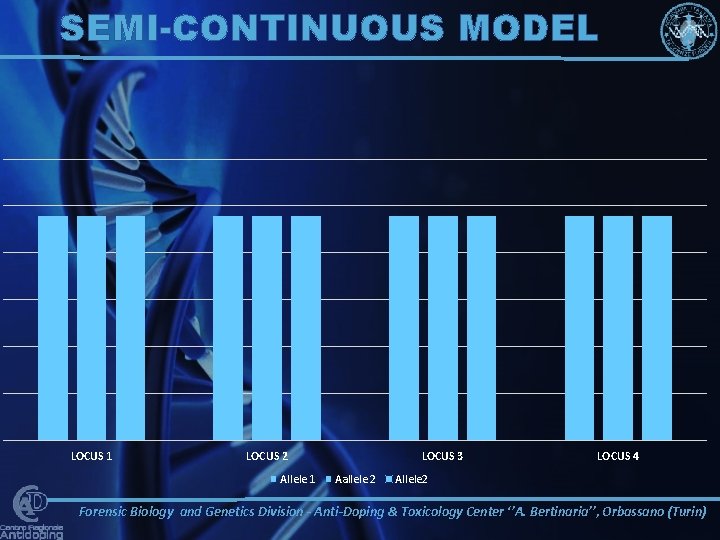
SEMI-CONTINUOUS MODEL LOCUS 1 LOCUS 2 Allele 1 LOCUS 3 Aallele 2 LOCUS 4 Allele 2 Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

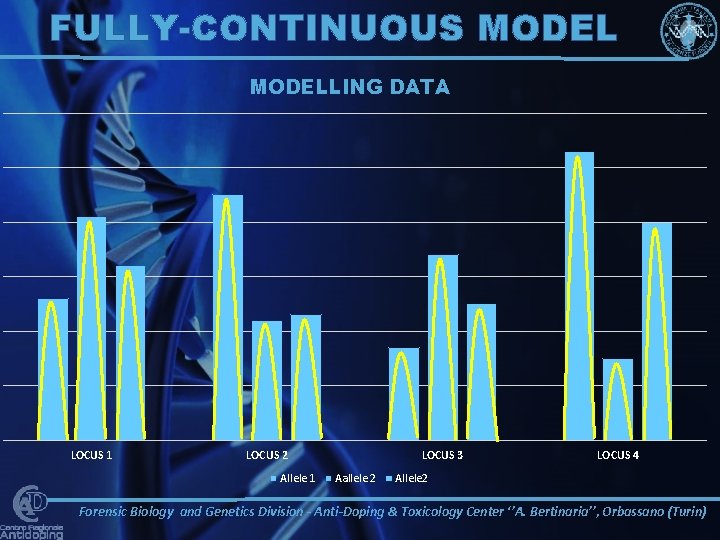
FULLY-CONTINUOUS MODELLING DATA LOCUS 1 LOCUS 2 Allele 1 LOCUS 3 Aallele 2 LOCUS 4 Allele 2 Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

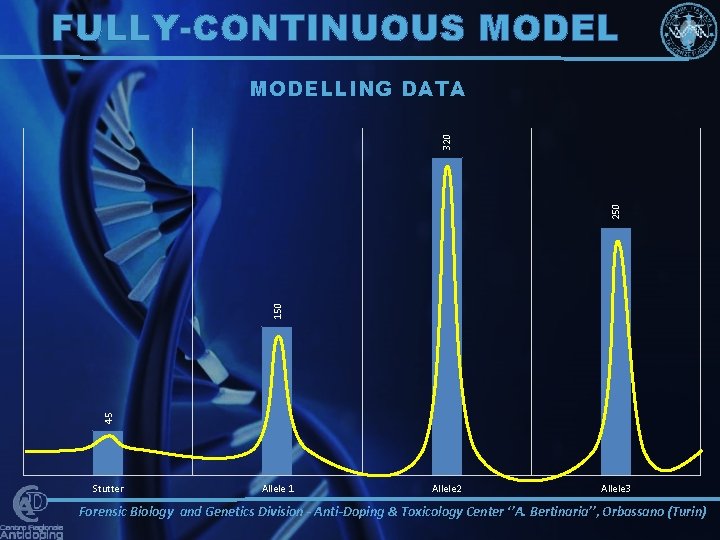
FULLY-CONTINUOUS MODEL 45 150 250 320 MODELLING DATA Stutter Allele 1 Allele 2 Allele 3 Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

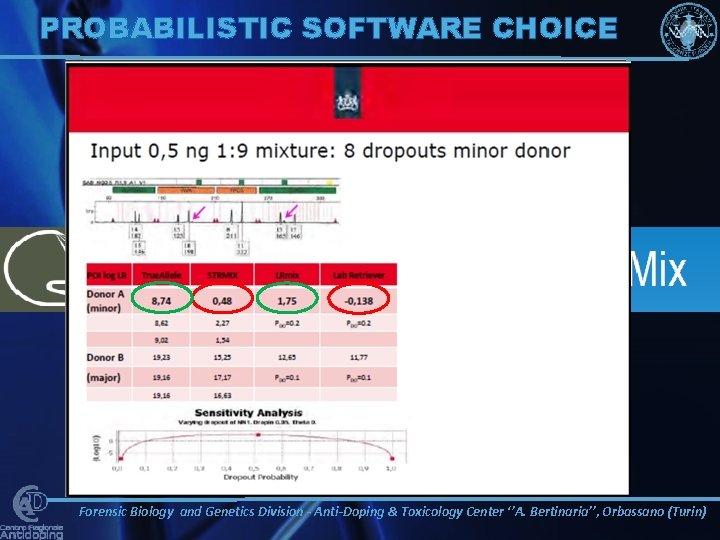
C R O C H C R O S SS S EC K PROBABILISTIC SOFTWARE CHOICE E C K Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

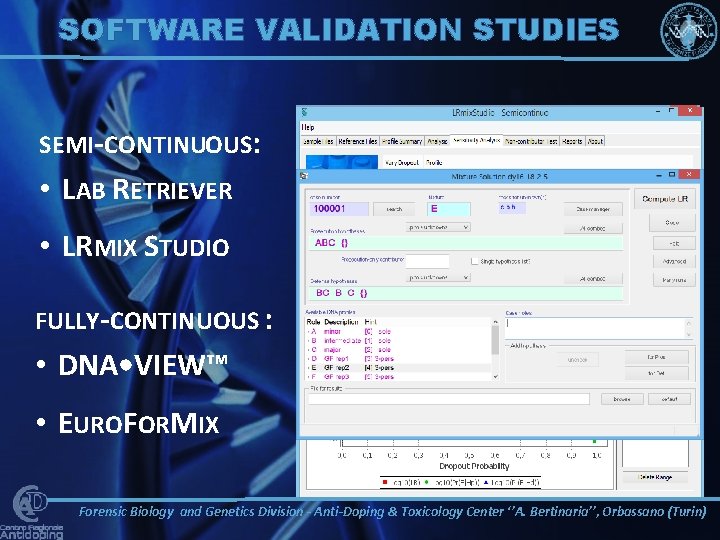
SOFTWARE VALIDATION STUDIES SEMI-CONTINUOUS: • LAB RETRIEVER • LRMIX STUDIO FULLY-CONTINUOUS : • DNA • VIEW™ • EUROFORMIX Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

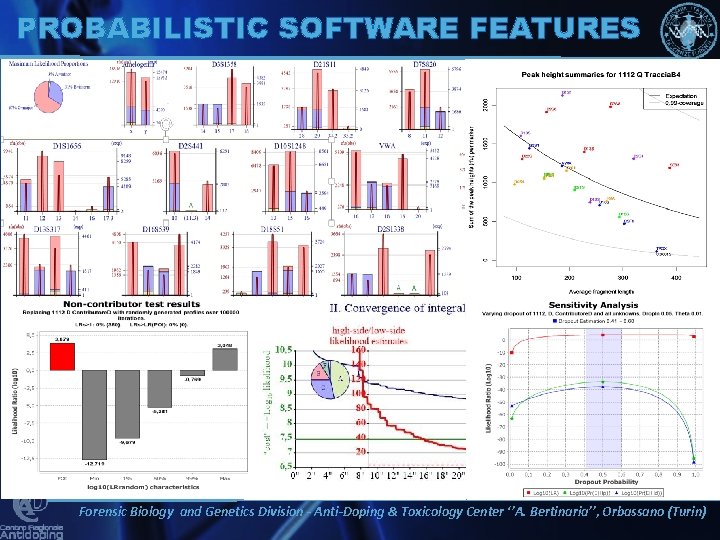
PROBABILISTIC SOFTWARE FEATURES Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

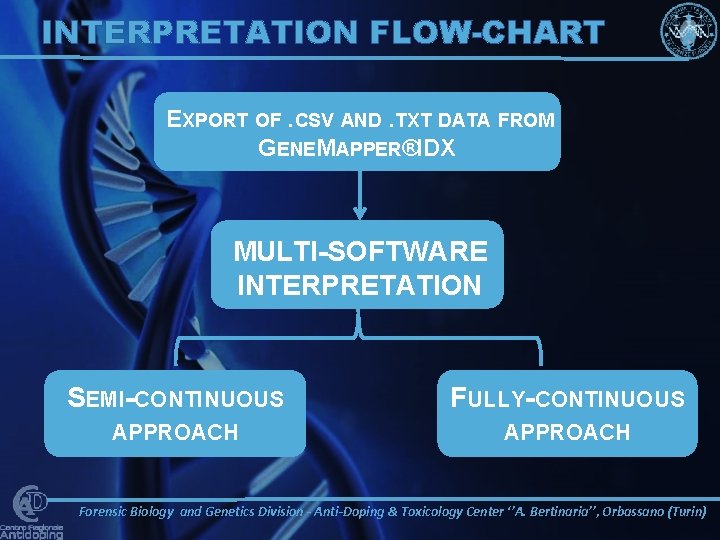
INTERPRETATION FLOW-CHART GENEMAPPER®ID-X ELECTROPHEROGRAM . HID DATA INTO OSIRIS. OAR DATA IMPORTED INTO ARMEDXPERTTM SOFTWARE ANALYTICAL THRESHOLD EVALUATION EXPORT OF. CSV AND. TXT DATA FROM GENEMAPPER®IDX Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

INTERPRETATION FLOW-CHART EXPORT OF. CSV AND. TXT DATA FROM GENEMAPPER®IDX MULTI-SOFTWARE INTERPRETATION SEMI-CONTINUOUS FULLY-CONTINUOUS APPROACH Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

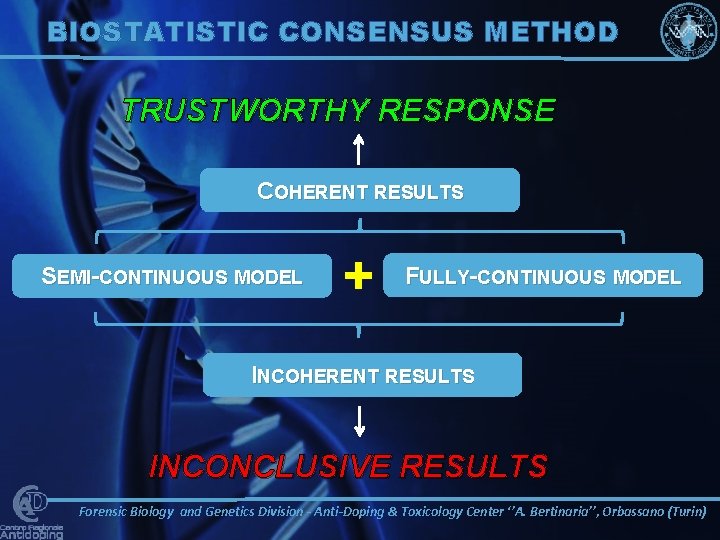
BIOSTATISTIC CONSENSUS METHOD TRUSTWORTHY RESPONSE COHERENT RESULTS SEMI-CONTINUOUS MODEL + FULLY-CONTINUOUS MODEL INCOHERENT RESULTS INCONCLUSIVE RESULTS Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

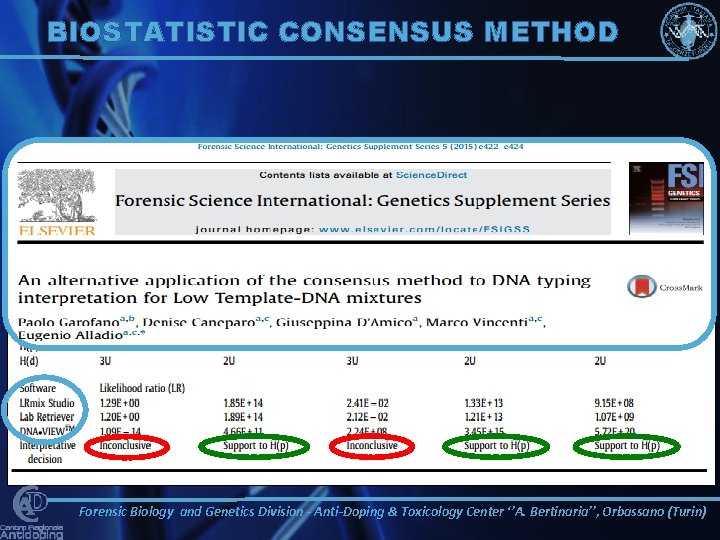
BIOSTATISTIC CONSENSUS METHOD Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

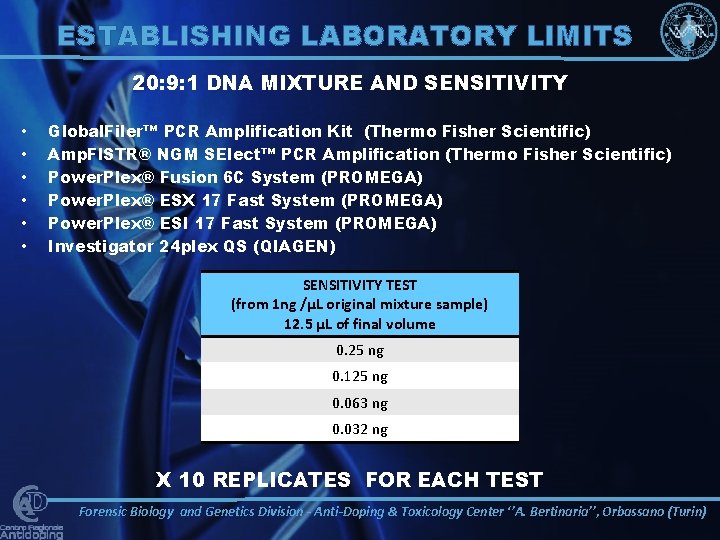
ESTABLISHING LABORATORY LIMITS 20: 9: 1 DNA MIXTURE AND SENSITIVITY • • • Global. Filer™ PCR Amplification Kit (Thermo Fisher Scientific) Amp. Fl. STR® NGM SElect™ PCR Amplification (Thermo Fisher Scientific) Power. Plex® Fusion 6 C System (PROMEGA) Power. Plex® ESX 17 Fast System (PROMEGA) Power. Plex® ESI 17 Fast System (PROMEGA) Investigator 24 plex QS (QIAGEN) SENSITIVITY TEST (from 1 ng /μL original mixture sample) 12. 5 μL of final volume 0. 25 ng 0. 125 ng 0. 063 ng 0. 032 ng X 10 REPLICATES FOR EACH TEST Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

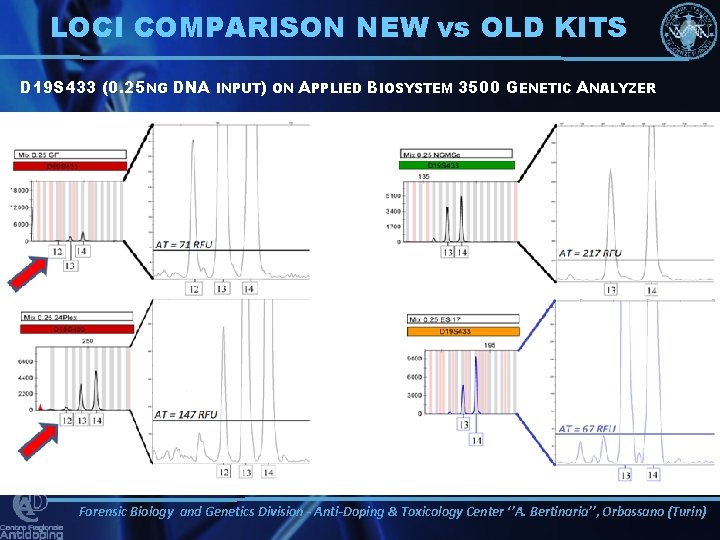
LOCI COMPARISON NEW vs OLD KITS D 19 S 433 (0. 25 NG DNA INPUT) ON APPLIED BIOSYSTEM 3500 GENETIC ANALYZER Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

LOCI COMPARISON NEW vs OLD KITS D 1 S 1656 (0. 032 NG DNA INPUT) ON APPLIED BIOSYSTEM 3500 GENETIC ANALYZER Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

LOCI COMPARISON ABI 3500 VS 3130 xl PROMEGA POWER PLEX ESX 17 - D 8 S 1179 (0. 032 ng DNA input) 3500 3130 Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

ESTABLISHING LABORATORY LIMITS 20: 9: 1 DNA MIXTURE AND DEGRADATION • • • Global. Filer™ PCR Amplification Kit (Thermo Fisher Scientific) Amp. Fl. STR® NGM SElect™ PCR Amplification (Thermo Fisher Scientific) Power. Plex® Fusion 6 C System (PROMEGA) Power. Plex® ESX 17 Fast System (PROMEGA) Power. Plex® ESI 17 Fast System (PROMEGA) Investigator 24 plex QS (QIAGEN) DEGRADATION TEST (from 1 ng/μL original mixture sample) D. I. = 2. 5 (40 min incubation at 90°) D. I. = 4. 5 (40 min + 20 min incubation at 90°) D. I. = 8 (40 min + 40 min incbutation at 90°) X 10 REPLICATES FOR EACH TEST Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

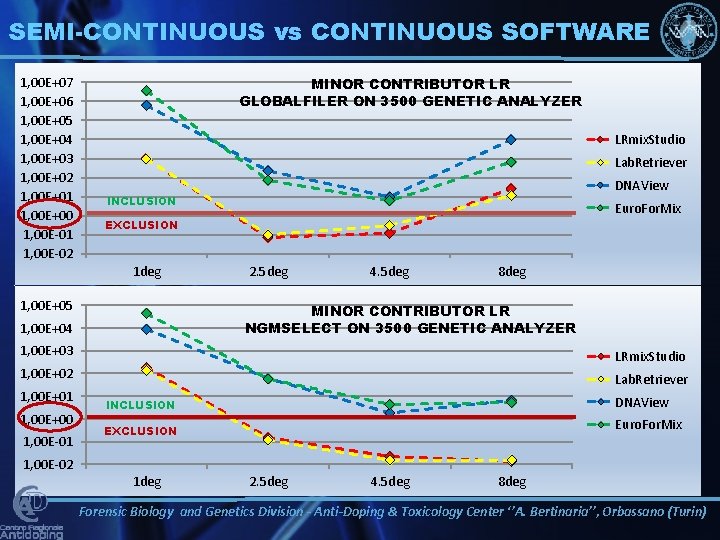
SEMI-CONTINUOUS vs CONTINUOUS SOFTWARE 1, 00 E+07 1, 00 E+06 1, 00 E+05 1, 00 E+04 1, 00 E+03 1, 00 E+02 1, 00 E+01 1, 00 E+00 1, 00 E-01 1, 00 E-02 MINOR CONTRIBUTOR LR GLOBALFILER ON 3500 GENETIC ANALYZER LRmix. Studio Lab. Retriever DNAView INCLUSION Euro. For. Mix EXCLUSION 1 deg 1, 00 E+05 2. 5 deg 4. 5 deg 8 deg MINOR CONTRIBUTOR LR NGMSELECT ON 3500 GENETIC ANALYZER 1, 00 E+04 1, 00 E+03 LRmix. Studio 1, 00 E+02 Lab. Retriever 1, 00 E+01 1, 00 E+00 1, 00 E-01 INCLUSION DNAView EXCLUSION Euro. For. Mix 1, 00 E-02 1 deg 2. 5 deg 4. 5 deg 8 deg Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

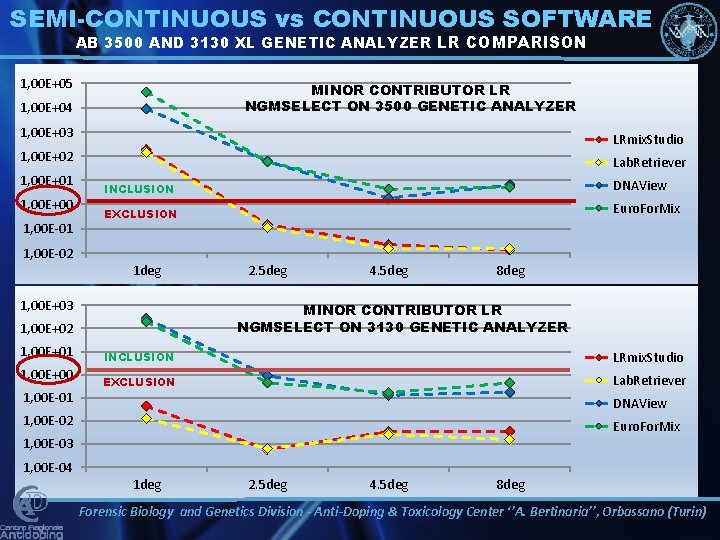
SEMI-CONTINUOUS vs CONTINUOUS SOFTWARE AB 3500 AND 3130 XL GENETIC ANALYZER LR COMPARISON 1, 00 E+05 MINOR CONTRIBUTOR LR NGMSELECT ON 3500 GENETIC ANALYZER 1, 00 E+04 1, 00 E+03 LRmix. Studio 1, 00 E+02 1, 00 E+01 1, 00 E+00 1, 00 E-01 Lab. Retriever INCLUSION DNAView EXCLUSION Euro. For. Mix 1, 00 E-02 1 deg 1, 00 E+03 1, 00 E+00 1, 00 E-01 4. 5 deg 8 deg MINOR CONTRIBUTOR LR NGMSELECT ON 3130 GENETIC ANALYZER 1, 00 E+02 1, 00 E+01 2. 5 deg INCLUSION LRmix. Studio EXCLUSION Lab. Retriever DNAView 1, 00 E-02 Euro. For. Mix 1, 00 E-03 1, 00 E-04 1 deg 2. 5 deg 4. 5 deg 8 deg Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

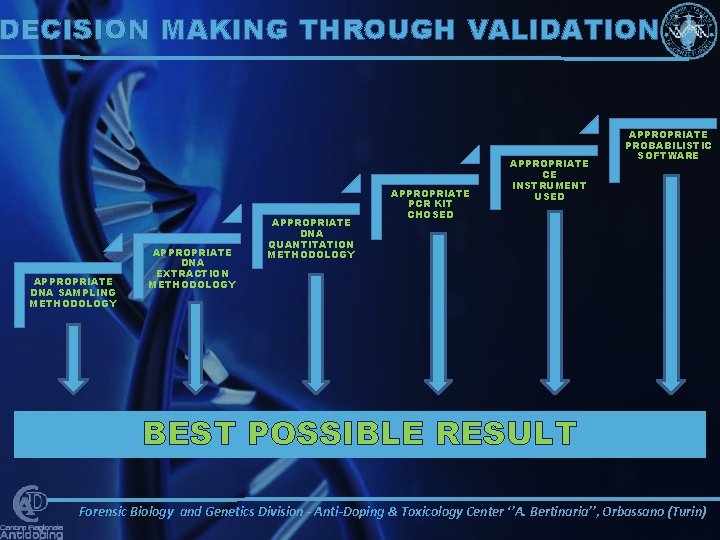
DECISION MAKING THROUGH VALIDATION APPROPRIATE DNA SAMPLING METHODOLOGY APPROPRIATE DNA EXTRACTION METHODOLOGY APPROPRIATE DNA QUANTITATION METHODOLOGY APPROPRIATE PCR KIT CHOSED APPROPRIATE CE INSTRUMENT USED APPROPRIATE PROBABILISTIC SOFTWARE BEST POSSIBLE RESULT Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

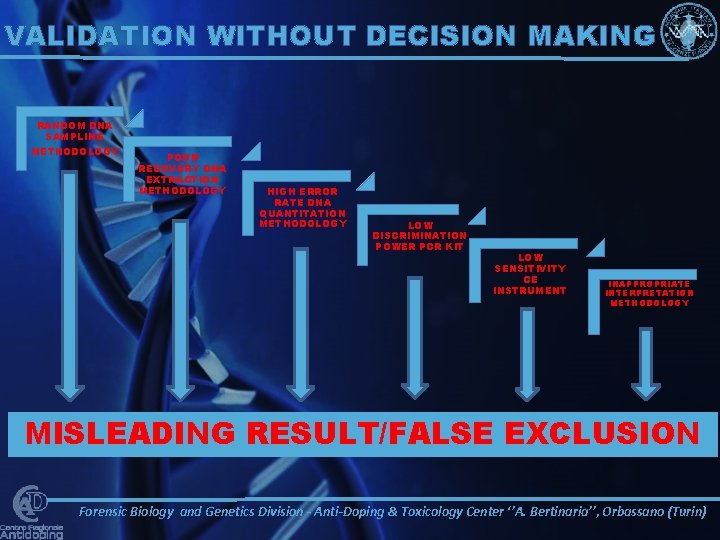
VALIDATION WITHOUT DECISION MAKING RANDOM DNA SAMPLING METHODOLOGY POOR RECOVERY DNA EXTRACTION METHODOLOGY HIGH ERROR RATE DNA QUANTITATION METHODOLOGY LOW DISCRIMINATION POWER PCR KIT LOW SENSITIVITY CE INSTRUMENT INAPPROPRIATE INTERPRETATION METHODOLOGY MISLEADING RESULT/FALSE EXCLUSION Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

CONCLUSIONS • PROBABILISTIC GENOTIPING ISO 17025 ACCREDITION NEED A DEFINITON OF YOUR METHOD’S SCOPE AND VARIABLES DURING ASSESSMENT • SPECIFIC VALIDATION STUDIES HAVE TO BE DONE IN ORDER TO ESTABLISH LABORATORY LIMITS ON TESTS PERFORMED • FOR EACH ANALITICAL STEP USED TO OBTAIN RESULTS, UNCERTAINTY OF MEASUREMENT HAS TO BE CALCULATD AND TAKEN INTO ACCOUNT • STATISTICAL MODELS USED FOR PROBABILISTIC GENOTYPING HAVE TO BE COMPREHENDED AND SOFTWARE VALIDATED BY CROSS-COMPARISON IN ORDER TO CARRY OUT RELIABLE RESULTS IN ALL CONDITIONS NEEDED • DATA USED FOR MIXTURE INTERPRETATION ARE THE OUTCOME OF A NUMBER OF SEPARATE METHODOLOGIES WHICH CAN STRONGLY AFFECT PROBABILISTIC GENOTYPING RESULTS • MULTI-SOFTWARE APPROACH MAY REPRESENT A POWERFUL TOOL BOTH FOR ACCREDITATION AND COMPLEX DNA MIXTURE INTERPRETATION Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

ACKNOWLEDGMENTS BRUCE BUDOWLE – NORTH TEXAS UNIVERSITY CHARLES BRENNER – BERKELEY UNIVERSITY LUIGI ARMOGIDA – NICHEVISION GEORGE RILEY – NCBI ROBERT GOORE – NCBI TIM KALAFUT – US ARMY CRIME LABORATORY JOEL SUTTON – US ARMY CRIME LABORATORY CORINA CG BENSCHOP – NETHERLANDS FORENSIC INTITUTE OVYIND BLEKA – NORWEGIAN INSTITUTE OF PUBLIC HEALTH LUCIANO GAROFANO – ITALIAN ACADEMY OF FORENSIC SCIENCES Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

ACKNOWLEDGMENTS LABORATORY STAFF MONICA OMEDEI SELENA CISANA GIUSY D’AMICO DENISE CANEPARO VERONICA MORRA EUGENIO ALLADIO Forensic Biology and Genetics Division - Anti-Doping & Toxicology Center ‘’A. Bertinaria’’, Orbassano (Turin)

Speaker was provided travel and hotel support by Thermo Fisher Scientific for this presentation, but no remuneration When used for purposes other than Human Identification or Paternity Testing the instruments and software modules cited are for Research Use Only. Not for use in diagnostic procedures. Thermo Fisher Scientific and its affiliates are not endorsing, recommending, or promoting any use or application of Thermo Fisher Scientific products presented by third parties during this seminar. Information and materials presented or provided by third parties are provided as-is and without warranty of any kind, including regarding intellectual property rights and reported results. Parties presenting images, text and material represent they have the rights

THANKS FOR YOUR ATTENTION paologarofano@hotmail. com
 Isoiec
Isoiec Uni cei en iso 50001:2018
Uni cei en iso 50001:2018 Incertezza estesa definizione
Incertezza estesa definizione Iso 17025 environmental conditions
Iso 17025 environmental conditions Como se escribe 17025
Como se escribe 17025 Exemple pratique de la norme iso 17025
Exemple pratique de la norme iso 17025 Iso 17025:2007
Iso 17025:2007 Ntp 17025
Ntp 17025 Daftar pertanyaan audit internal iso 17025:2017
Daftar pertanyaan audit internal iso 17025:2017 Ntp 17025
Ntp 17025 Ntp iso 17025
Ntp iso 17025 55 miller street
55 miller street National board of accreditation questions and answers
National board of accreditation questions and answers Accreditation board for engineering and technology
Accreditation board for engineering and technology Cei format
Cei format Cei metronorte
Cei metronorte Cei 7 regi legendari ai romei
Cei 7 regi legendari ai romei Rudy is writing an essay
Rudy is writing an essay 64 12
64 12 Opera si viata lui mihai eminescu
Opera si viata lui mihai eminescu Ferice de cei flamanzi si insetati dupa neprihanire
Ferice de cei flamanzi si insetati dupa neprihanire Ferice de cei ce plang
Ferice de cei ce plang Cei (comunidade dos estados independentes slides)
Cei (comunidade dos estados independentes slides) Cei paragraph examples
Cei paragraph examples Cei doi martori ai apocalipsei
Cei doi martori ai apocalipsei Clasici ai literaturii romane
Clasici ai literaturii romane Fericiti facatorii de pace
Fericiti facatorii de pace Trish collins detention
Trish collins detention Cei with food
Cei with food Cei rekalde
Cei rekalde Peninsula italica roma
Peninsula italica roma Mihai eminescu viata si opera
Mihai eminescu viata si opera Marchio di qualità imq e marcatura ce
Marchio di qualità imq e marcatura ce Cei 23-3
Cei 23-3 Difference between nominative and accusative
Difference between nominative and accusative Staqc
Staqc Berkeley extension
Berkeley extension Accsc accreditation good or bad
Accsc accreditation good or bad Fibaa accreditation
Fibaa accreditation Ilac mra
Ilac mra Ea european accreditation
Ea european accreditation Acgme accreditation withheld
Acgme accreditation withheld