Ions and Ionic Charge Ions are atoms that

- Slides: 7

Ions and Ionic Charge • Ions are atoms that have an electrical charge (+/-) • Unstable atoms will want to bond with other unstable atoms; have an electrical charge • Stable atoms will not bond with any other atoms and cannot be charged ions

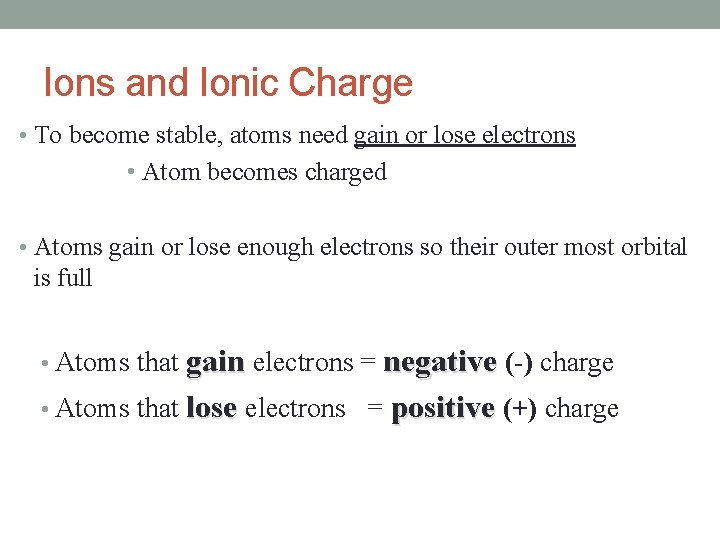
Ions and Ionic Charge • To become stable, atoms need gain or lose electrons • Atom becomes charged • Atoms gain or lose enough electrons so their outer most orbital is full • Atoms that gain electrons = negative (-) charge • Atoms that lose electrons = positive (+) charge

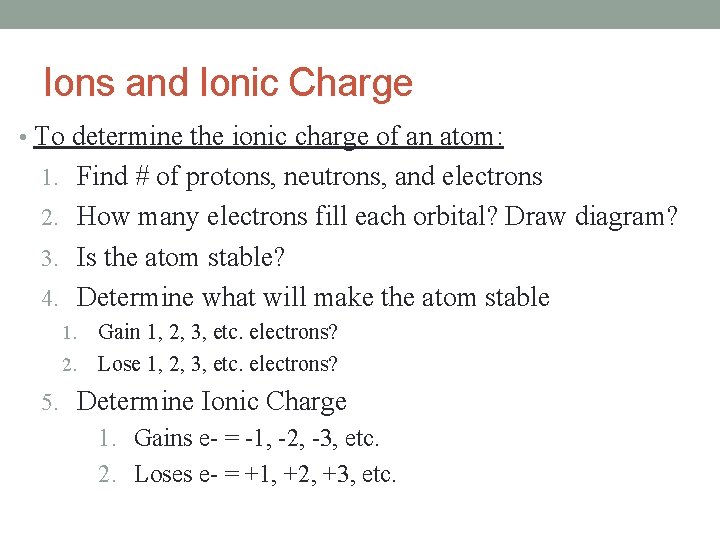
Ions and Ionic Charge • To determine the ionic charge of an atom: 1. Find # of protons, neutrons, and electrons 2. How many electrons fill each orbital? Draw diagram? 3. Is the atom stable? 4. Determine what will make the atom stable Gain 1, 2, 3, etc. electrons? 2. Lose 1, 2, 3, etc. electrons? 1. 5. Determine Ionic Charge 1. Gains e- = -1, -2, -3, etc. 2. Loses e- = +1, +2, +3, etc.

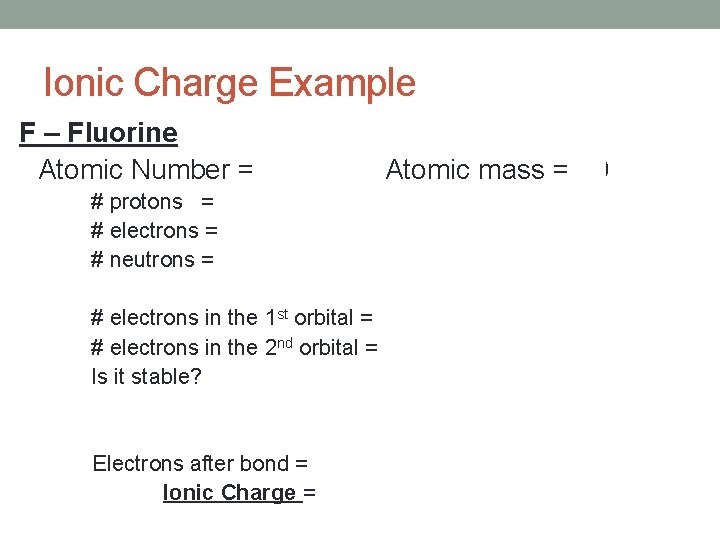
Ionic Charge Example F – Fluorine Atomic Number = 9 Atomic mass = 19 # protons = 9 # electrons = 9 # neutrons = 10 # electrons in the 1 st orbital = 2 # electrons in the 2 nd orbital = 7 Is it stable? No Electrons after bond = 10 It gains 1 e. Ionic Charge = -1 (Easier to gain 1 e-)

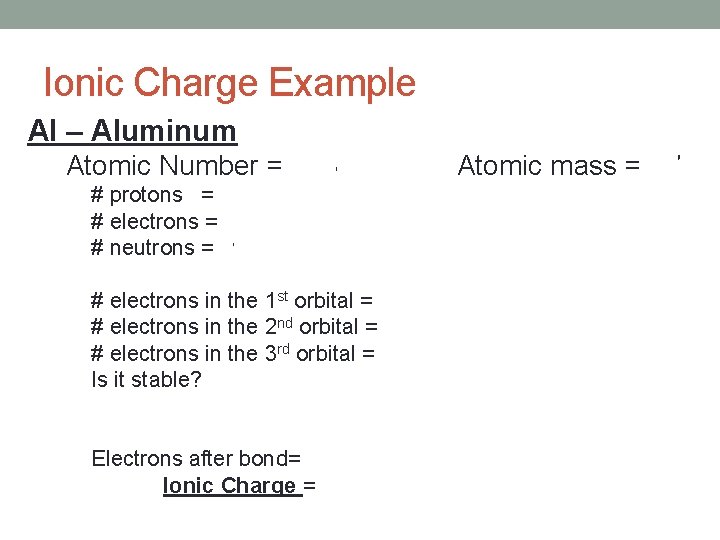
Ionic Charge Example Al – Aluminum Atomic Number = 13 Atomic mass = 27 # protons = 13 # electrons = 13 # neutrons = 14 # electrons in the 1 st orbital = 2 # electrons in the 2 nd orbital = 8 # electrons in the 3 rd orbital = 3 Is it stable? No Electrons after bond= 10 It loses 3 e. Ionic Charge = +3 (Easier to lose 3 e-)

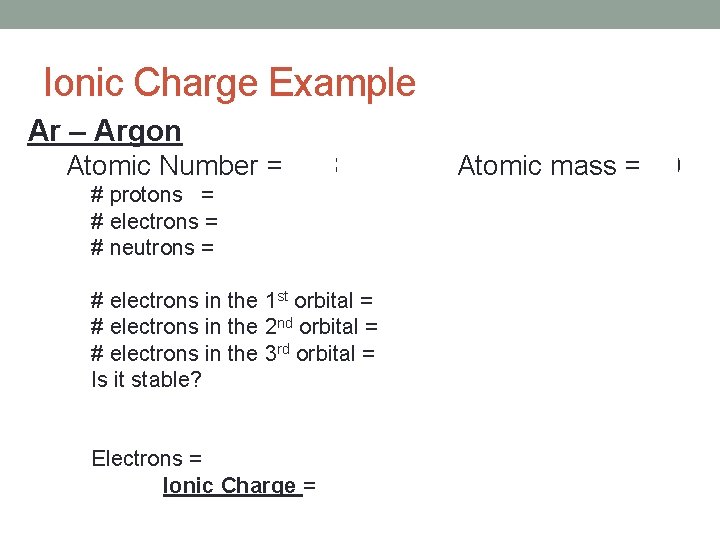
Ionic Charge Example Ar – Argon Atomic Number = 18 Atomic mass = 40 # protons = 18 # electrons = 18 # neutrons = 22 # electrons in the 1 st orbital = 2 # electrons in the 2 nd orbital = 8 # electrons in the 3 rd orbital = 8 Is it stable? Yes Electrons = 18 Ionic Charge = 0 (Outer orbital is full)

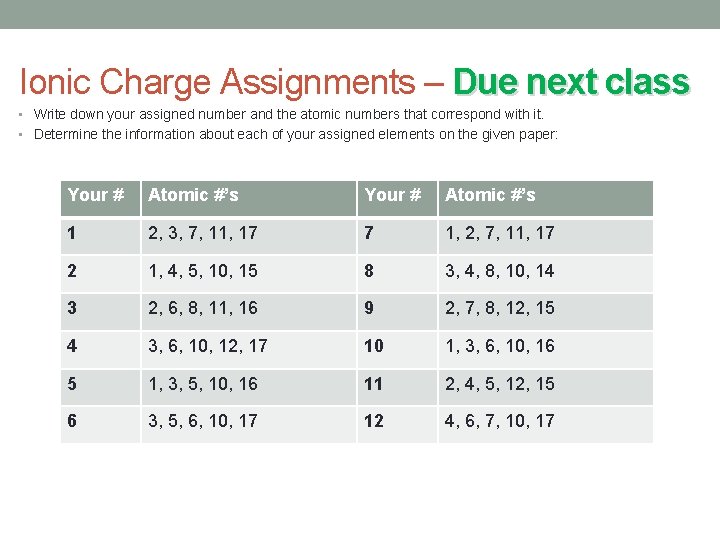
Ionic Charge Assignments – Due next class • Write down your assigned number and the atomic numbers that correspond with it. • Determine the information about each of your assigned elements on the given paper: Your # Atomic #’s 1 2, 3, 7, 11, 17 7 1, 2, 7, 11, 17 2 1, 4, 5, 10, 15 8 3, 4, 8, 10, 14 3 2, 6, 8, 11, 16 9 2, 7, 8, 12, 15 4 3, 6, 10, 12, 17 10 1, 3, 6, 10, 16 5 1, 3, 5, 10, 16 11 2, 4, 5, 12, 15 6 3, 5, 6, 10, 17 12 4, 6, 7, 10, 17