INTRODUCTION TO MATERIAL SCIENCE AND ENGINEERING Miss NOORHIDAYAH









- Slides: 17

INTRODUCTION TO MATERIAL SCIENCE AND ENGINEERING Miss NOORHIDAYAH ISHAK

By the end of this chapter student would be able to: • Explain the different between materials science & materials engineering • List primary classifications of materials and mention their distinctive properties • Explain categories of advanced materials

What are Materials? • Materials may be defined as substance of which something is composed or made. • We obtain materials from earth crust and atmosphere. • Examples : Silicon and Iron constitute 27. 72 and 5. 00 percentage of weight of earths crust respectively. Nitrogen and Oxygen constitute 78. 08 and 20. 95 percentage of dry air by volume respectively. 2

Why the Study of Materials is Important • Production and processing of materials constitute a large part of our economy. • Engineers choose materials to suite design. • New materials might be needed for some new applications. • Modification of properties might be needed for some applications. • Engineers in all discipline should have some basic and applied knowledge of engineering materials. 3

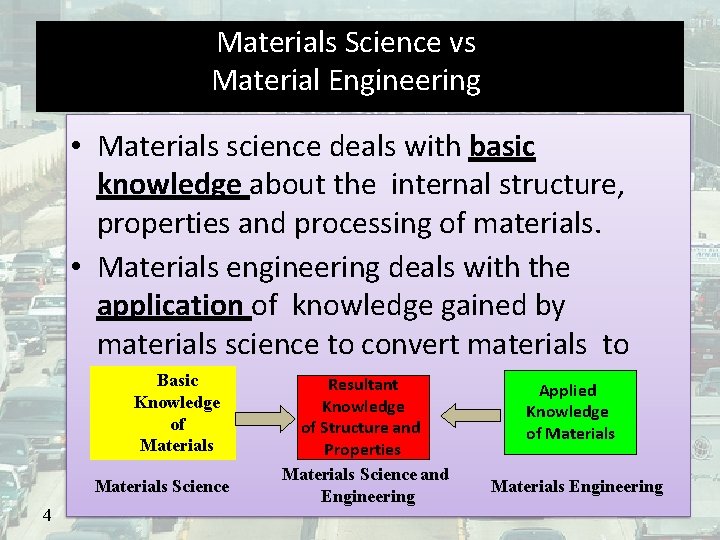
Materials Science vs Material Engineering • Materials science deals with basic knowledge about the internal structure, properties and processing of materials. • Materials engineering deals with the application of knowledge gained by materials science to convert materials to Basic Resultant products. Applied Knowledge of Materials Science 4 Knowledge of Structure and Properties Materials Science and Engineering Knowledge of Materials Engineering

Types of Materials POLYMERIC METALLIC CERAMIC

METALLIC Inorganic substances that composed of one or more metallic elements and may also contain some nonmetallic elements. Metallic elements: - Iron, Copper, Aluminum, Ni, and titanium. Non metallic elements: -Carbon, Nitrogen, Oxygen Metallic element may combine with nonmetallic elements. Example: - Silicon Carbide, Iron Oxide. Have crystalline structure. Good thermal and electric conductors. 6

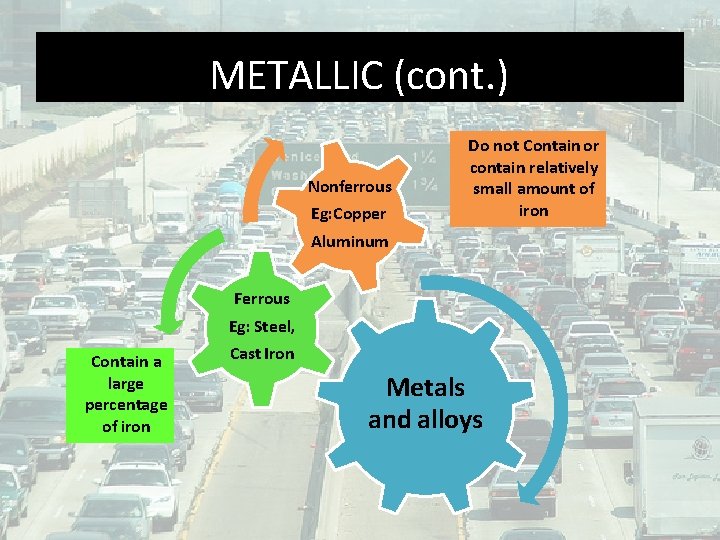
METALLIC (cont. ) Nonferrous Eg: Copper Do not Contain or contain relatively small amount of iron Aluminum Ferrous Eg: Steel, Contain a large percentage of iron Cast Iron Metals and alloys

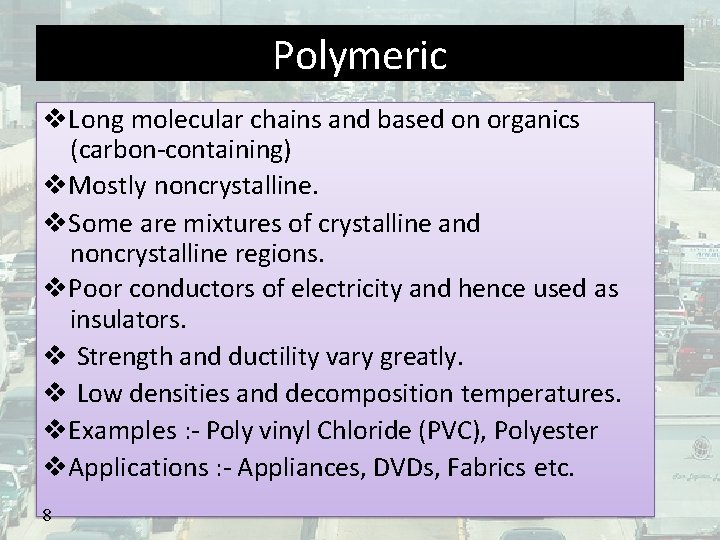
Polymeric Long molecular chains and based on organics (carbon-containing) Mostly noncrystalline. Some are mixtures of crystalline and noncrystalline regions. Poor conductors of electricity and hence used as insulators. Strength and ductility vary greatly. Low densities and decomposition temperatures. Examples : - Poly vinyl Chloride (PVC), Polyester Applications : - Appliances, DVDs, Fabrics etc. 8

CERAMICS Inorganic materials (metallic and nonmetallic) which chemically bonded together. Can be crystalline, noncrystalline or mixture of both. High hardness, strength and wear resistance. Very good insulator. Hence used for furnace lining for heat treating and melting metals. Other applications : Abrasives, construction materials, utensils etc. Example: - Porcelain, Glass, Silicon nitride.

Types of Materials ELECTRONIC COMPOSITE

COMPOSITE Mixture of two or more materials. Consists of a filler material and a binding material. Materials only bond, will not dissolve in each other. Mainly two types : Fibrous: Fibers in a matrix Particulate: Particles in a matrix Matrix can be metals, ceramic or polymer Examples : -Fiber Glass ( Reinforcing material in a polyester or epoxy matrix)Concrete ( Gravels or steel rods reinforced in cement and sand) Applications: - Aircraft wings and engine construction.

ELECTRONIC Not Major by volume but very important for advanced engineering technology. Silicon is a common electronic material. Its electrical characteristics are changed by adding impurities. Examples: - Silicon chips, transistors Applications : - Computers, Integrated Circuits, Satellites etc. 12

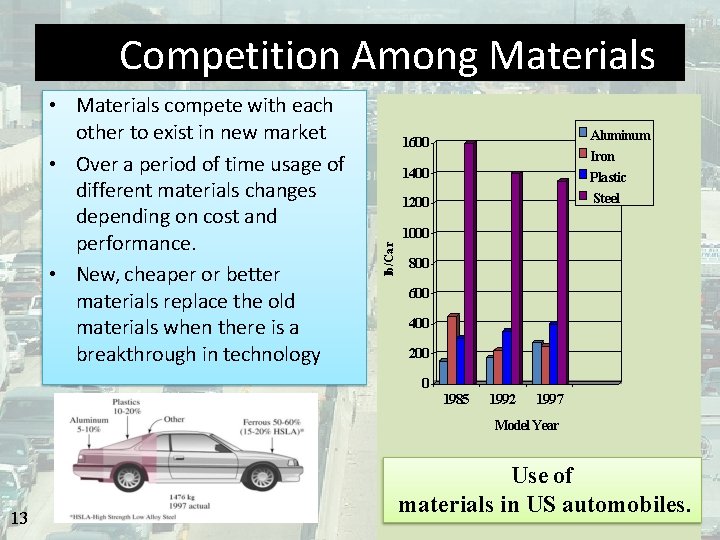
Competition Among Materials Example: 1600 1400 1200 Aluminum Iron Plastic Steel 1000 lb/Car • Materials compete with each other to exist in new market • Over a period of time usage of different materials changes depending on cost and performance. • New, cheaper or better materials replace the old materials when there is a breakthrough in technology 800 600 400 200 0 1985 1992 1997 Model Year 13 Use of materials in US automobiles.

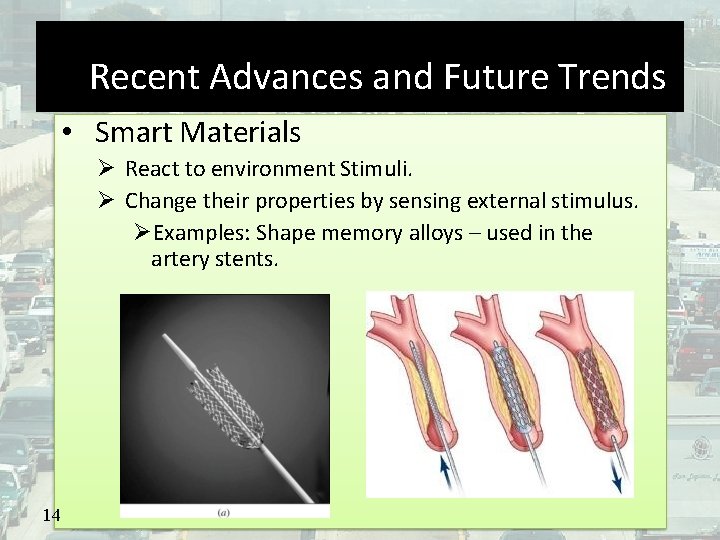
Recent Advances and Future Trends • Smart Materials React to environment Stimuli. Change their properties by sensing external stimulus. Examples: Shape memory alloys – used in the artery stents. 14

Recent Advances and Future Trends • Nanomaterials Smaller than 100 nm particle size. Materials have special properties. Very hard and strong characteristics. Research in progress. Example: Carbon nanofiber reinforced plastic: very light but stronger than metals. 15

THE END
 M tech structural engineering
M tech structural engineering Science fusion online
Science fusion online As a child, which subject of science was your favourite?
As a child, which subject of science was your favourite? Datum shift
Datum shift Ethnocentrism examples
Ethnocentrism examples Material and non material culture examples
Material and non material culture examples All groups create norms to enforce their cultural values.
All groups create norms to enforce their cultural values. Useful materials in science
Useful materials in science Material usage variance = material mix variance +
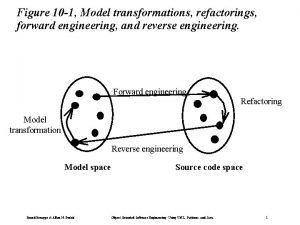
Material usage variance = material mix variance + Forward engineering in software engineering
Forward engineering in software engineering Mit material science
Mit material science Material science lab report
Material science lab report Microwaves definition science
Microwaves definition science What is material science
What is material science Materials science oxford
Materials science oxford Lee kong chian faculty of engineering and science
Lee kong chian faculty of engineering and science Ucf computer engineering
Ucf computer engineering Hot materials
Hot materials