Intranasal Delivery of Proteins Using Cationic Liposomes for



- Slides: 11

Intranasal Delivery of Proteins Using Cationic Liposomes for the Treatment of Parkinson’s Disease and the Use of Bioquant® Image Analysis Software Presented by Mattia M. Migliore April 20, 2007

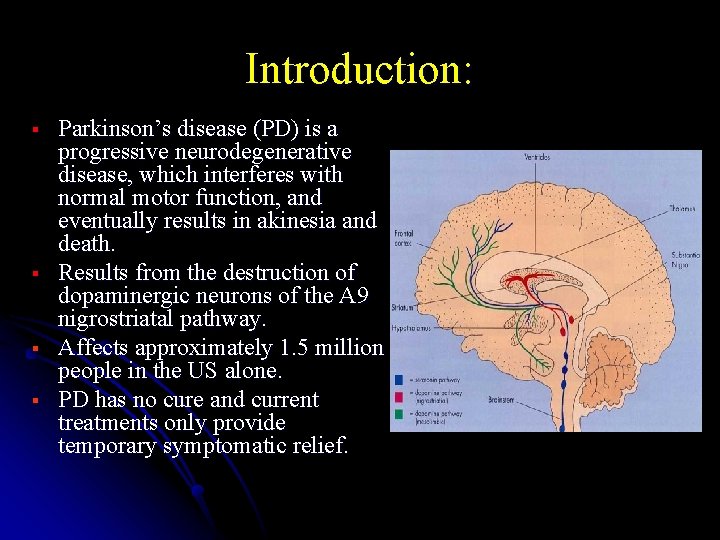
Introduction: § § Parkinson’s disease (PD) is a progressive neurodegenerative disease, which interferes with normal motor function, and eventually results in akinesia and death. Results from the destruction of dopaminergic neurons of the A 9 nigrostriatal pathway. Affects approximately 1. 5 million people in the US alone. PD has no cure and current treatments only provide temporary symptomatic relief.

Introduction (cont. ): § § GDNF is a protein with therapeutic potential for PD because it exerts neurotrophic and neuroregenerative effects of dopamine neurons. GDNF levels are decreased by as much as 19. 4% per SN neuron in PD patients (Chauhan et al. , 2001; Hurelbrink and Barker, 2004). § § GDNF does not cross the blood-brain barrier (BBB). GDNF administration requires invasive intracerebral infusions to reach its site of action.

Introduction (cont. ): § § The goal of this project is to develop a cationic liposomal drug delivery system to transport GDNF to the brain using the intranasal route of administration. The intranasal route of administration was chosen because it is non-invasive, and it bypasses the BBB.

Specific AIMS: § § § Specific AIM 1: To characterize and optimize a nanoparticle formulation for intranasal GDNF. Using first a model protein to optimize our cationic liposomal formulation. Specific AIM 2: To determine brain delivery of GDNF in rats following intranasal administration. Specific AIM 3: To determine therapeutic efficacy of intranasal GDNF in a rat model of Parkinson’s disease.

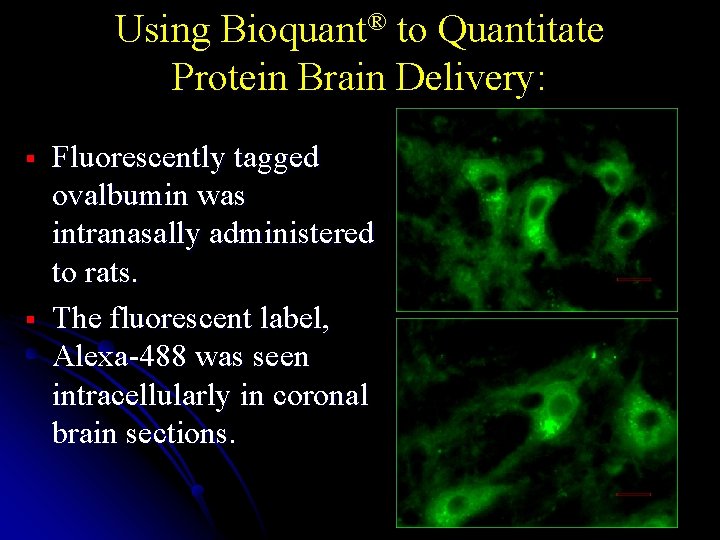
Using Bioquant® to Quantitate Protein Brain Delivery: § § Fluorescently tagged ovalbumin was intranasally administered to rats. The fluorescent label, Alexa-488 was seen intracellularly in coronal brain sections.

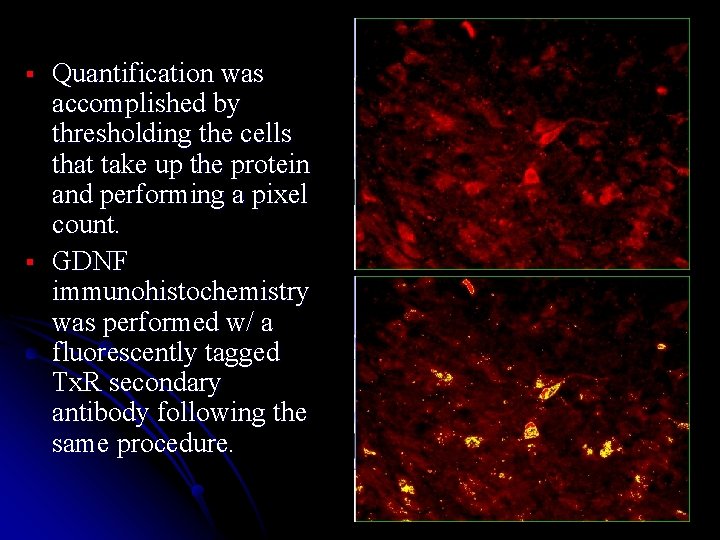
§ § Quantification was accomplished by thresholding the cells that take up the protein and performing a pixel count. GDNF immunohistochemistry was performed w/ a fluorescently tagged Tx. R secondary antibody following the same procedure.

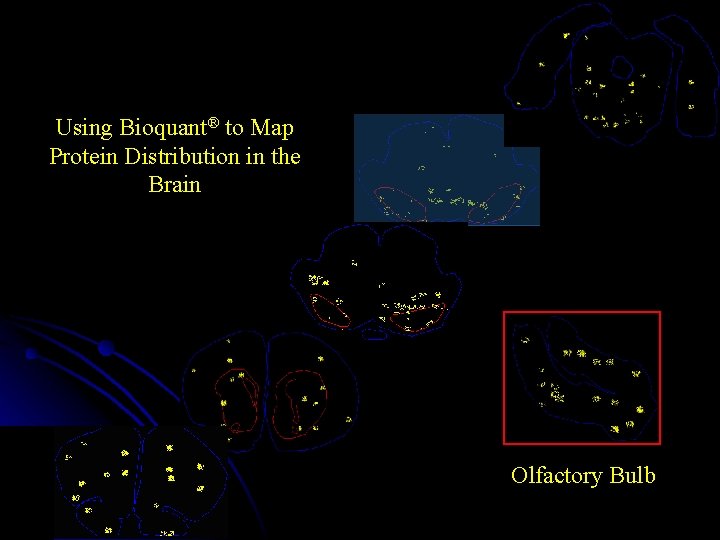
Using Bioquant® to Map Protein Distribution in the Brain Olfactory Bulb

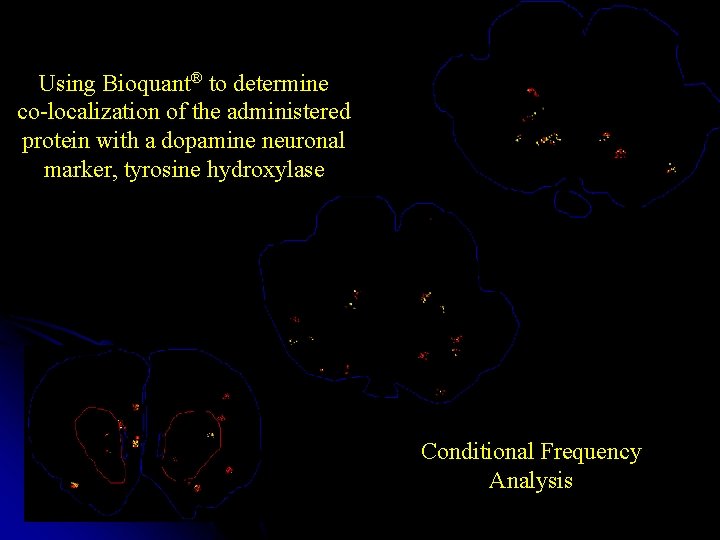
Using Bioquant® to determine co-localization of the administered protein with a dopamine neuronal marker, tyrosine hydroxylase Conditional Frequency Analysis

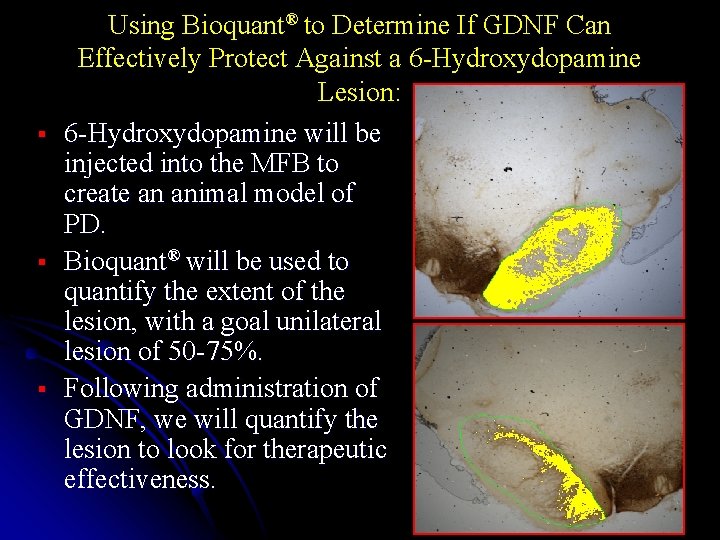
§ § § Using Bioquant® to Determine If GDNF Can Effectively Protect Against a 6 -Hydroxydopamine Lesion: 6 -Hydroxydopamine will be injected into the MFB to create an animal model of PD. Bioquant® will be used to quantify the extent of the lesion, with a goal unilateral lesion of 50 -75%. Following administration of GDNF, we will quantify the lesion to look for therapeutic effectiveness.

Conclusion: § Bioquant® will be used to qualitatively and quantitatively analyze the data in this project.