Initiation of Antiretroviral Therapy in Early Asymptomatic HIV


















- Slides: 36

Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection (The INSIGHT START Study Group) Presented by: Courtney Brown & Jeffery Quan March 24, 2016

Background - HIV (human immunodeficiency virus) is a 20 sided RNA virus that attacks our immune system by infecting CD 4+ immune cells Frequently replicates with minimal error checking Rates of HIV-complicated deaths increase as the number of CD 4+ T cells declines Initiation of antiretroviral therapy (ART) is usually deferred until a certain threshold level (CD 4+ count of 200 -350 cells per cubic millimeter) 66% of people with HIV are not receiving antiretroviral therapy HIV is transmitted through a number of different ways

Background- Other Trials Effect of Early versus Deferred Antiretroviral Therapy for HIV on Survival (2009) Compared relative risk of death was between two treatment groups as 351 -500 cells/mm 3 and >500 cells/mm 3 Increased risk of death in deferred therapy group defined as 351 -500 cells/mm 3 When to Initiate Combined Antiretroviral Therapy to Reduce Mortality and AIDSDefining Illness in HIV-Infected Persons in Developed Countries (2011) Observational studies have determined that mortality did not vary substantially from ART between >500 cells/mm 3 and 300 -500 cells/mm 3 Most studies have focused only on the risks of the acquired immunodeficiency syndrome (AIDS) and death and have not fully addressed the risks and benefits of initiating antiretroviral therapy in patients with a high CD 4+ count. Some studies have raised concern about the adverse effects of ART on cardiovascular and renal disease, particularly in older patients Kitahata MM, et al. N Engl J Med. 2009; 360(18): 1815 -26. Cain LE, et al. Ann Intern Med. 2011 Apr 19; 154(8): 509 -15.

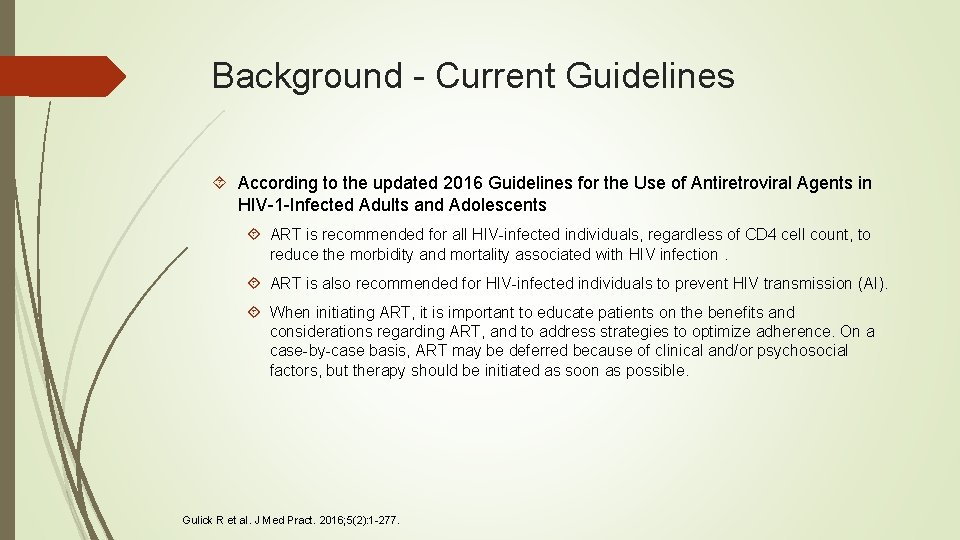
Background - Current Guidelines According to the updated 2016 Guidelines for the Use of Antiretroviral Agents in HIV-1 -Infected Adults and Adolescents ART is recommended for all HIV-infected individuals, regardless of CD 4 cell count, to reduce the morbidity and mortality associated with HIV infection. ART is also recommended for HIV-infected individuals to prevent HIV transmission (AI). When initiating ART, it is important to educate patients on the benefits and considerations regarding ART, and to address strategies to optimize adherence. On a case-by-case basis, ART may be deferred because of clinical and/or psychosocial factors, but therapy should be initiated as soon as possible. Gulick R et al. J Med Pract. 2016; 5(2): 1 -277.

Objective To determine the risks and benefits of the immediate initiation of ART in asymptomatic HIV-positive patients who have a CD 4+ count of >500 cells/mm 3, as compared with deferring initiation until the CD 4+ count is 350 cells/mm 3 Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

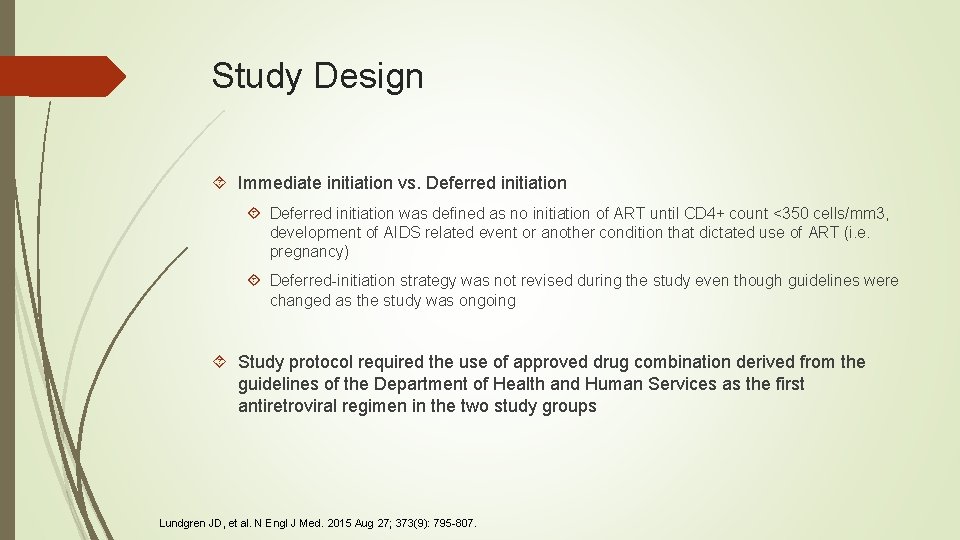
Study Design Immediate initiation vs. Deferred initiation was defined as no initiation of ART until CD 4+ count <350 cells/mm 3, development of AIDS related event or another condition that dictated use of ART (i. e. pregnancy) Deferred-initiation strategy was not revised during the study even though guidelines were changed as the study was ongoing Study protocol required the use of approved drug combination derived from the guidelines of the Department of Health and Human Services as the first antiretroviral regimen in the two study groups Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Study Design Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

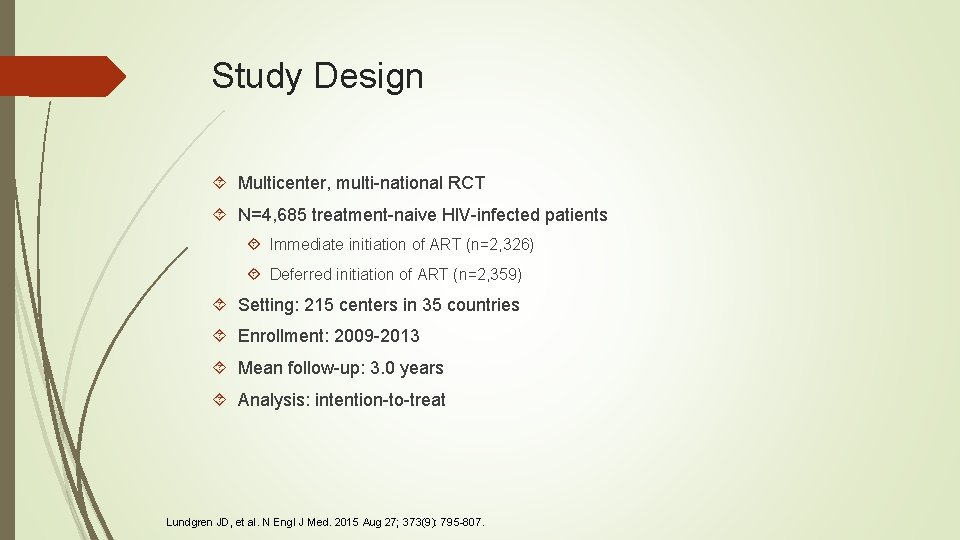
Study Design Multicenter, multi-national RCT N=4, 685 treatment-naive HIV-infected patients Immediate initiation of ART (n=2, 326) Deferred initiation of ART (n=2, 359) Setting: 215 centers in 35 countries Enrollment: 2009 -2013 Mean follow-up: 3. 0 years Analysis: intention-to-treat Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Inclusion Criteria HIV-positive infected patients Age ≥ 18 years Antiretroviral treatment-naive No progression to AIDS in “generally” good health Two CD 4+ counts >500 cells/mm 3, at least 2 weeks apart within 60 days before enrollment Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

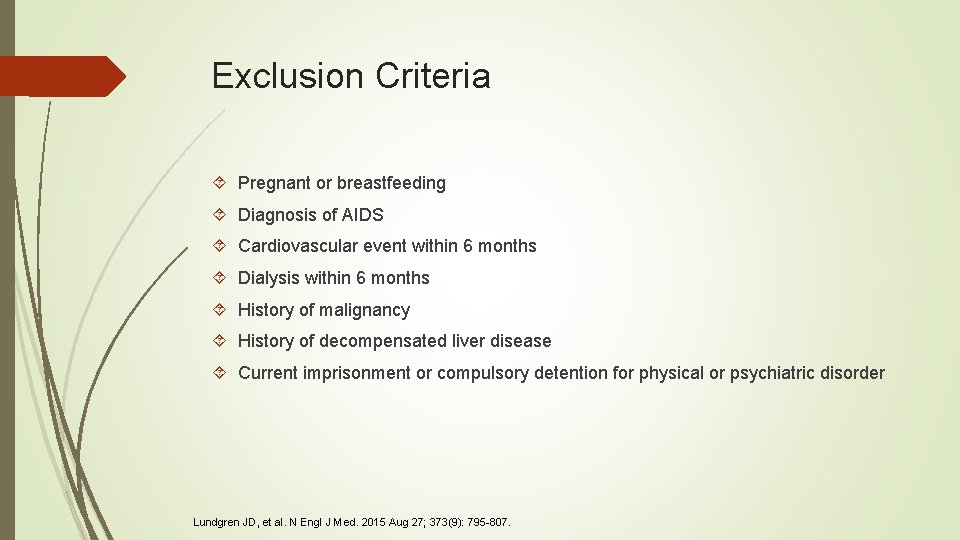
Exclusion Criteria Pregnant or breastfeeding Diagnosis of AIDS Cardiovascular event within 6 months Dialysis within 6 months History of malignancy History of decompensated liver disease Current imprisonment or compulsory detention for physical or psychiatric disorder Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Primary Endpoint was a composite outcome that included 2 major components Any serious AIDS related event, including death from AIDS and Hodgkin’s lymphoma Exceptions: nonfatal HSV infection and esophageal candidiasis were not counted because of their lesser severity. Any serious non-AIDS related event, including death from causes other than AIDS CV disease (MI, stroke or coronary revascularization) or death from CV disease, ESRD (initiation of dialysis or renal transplantation), or death from renal disease, liver disease (decompensated liver disease), non AIDS defining cancer (except for basal-cell or squamous-cell skin cancer) or death from cancer and death from any cause not attributable to AIDS An end-point review committee whose members were unaware of study-group assignments reviewed all reported ADEs. Events considered to be confirmed or probable were counted as end points Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Secondary Endpoints Serious AIDS related events Serious non-AIDS related events Death from any cause Grade 4 events Defined as potentially life-threatening symptomatic events not attributable to AIDS that required a medical intervention Unscheduled hospitalizations for reasons other than AIDS Grade 4 events and unscheduled hospitalizations were reported regardless of ART use or any perceived association with ART Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Study Method Patients were randomly assigned to either immediate ART or deferred ART Randomization was stratified by clinical site Assignments were obtained from a web-based program that verified participant eligibility Due to the nature of the study, investigators and participants were not blinded to the treatment group assignment. However, endpoints were reviewed blinded to treatment group Drugs that were primarily used for initial treatment in the immediate initiation group and the deferred-initiation group were: Tenofovir (89% in both groups) Emtricitabine (89% and 88%) Efavirenz (73% and 51%) Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Interim Monitoring Study investigators were unaware of interim summary results throughout the study On May 15, 2015, the board informed the National Institute of Allergy and Infectious Diseases (NIAID) and the study leadership that the primary question of the study had been answered and recommended that the findings be immediately disseminated Board also recommended offering ART to patients not currently receiving ART and continuing to follow these study patients 60% of the planned 213 primary events had occurred by the time of the review On May 27, 2015 the study team leaders and NIAID notified investigators and patients of the study findings Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Statistical Analysis Intention-to-treat Time-to-event methods were used to compare the two groups for primary end point, its two major components, death from any cause and other serious clinical events Kaplan-Meier survival curves Cox proportional-hazards model O’Brien-Fleming boundary and the Lan-De. Mets spending function based on information time (fraction of primary events accrued) were used to adjust for type I error in the analysis of the primary end point Sensitivity analyses were performed to assess the effects of the diagnostic certainty of events and of missing data Kaplan-Meier estimates were used to describe the time until initiation of ART All P values are two-sided Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

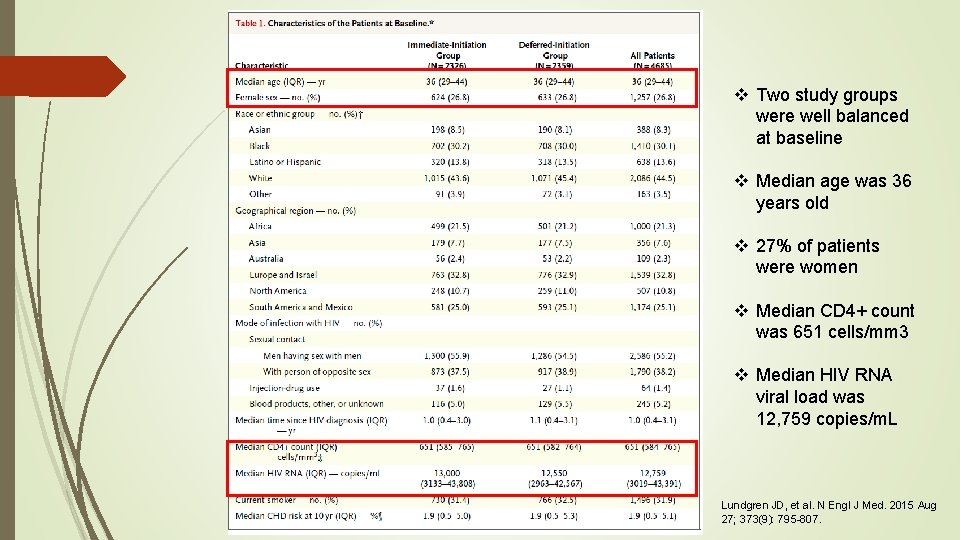
v Two study groups were well balanced at baseline v Median age was 36 years old v 27% of patients were women v Median CD 4+ count was 651 cells/mm 3 v Median HIV RNA viral load was 12, 759 copies/m. L Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

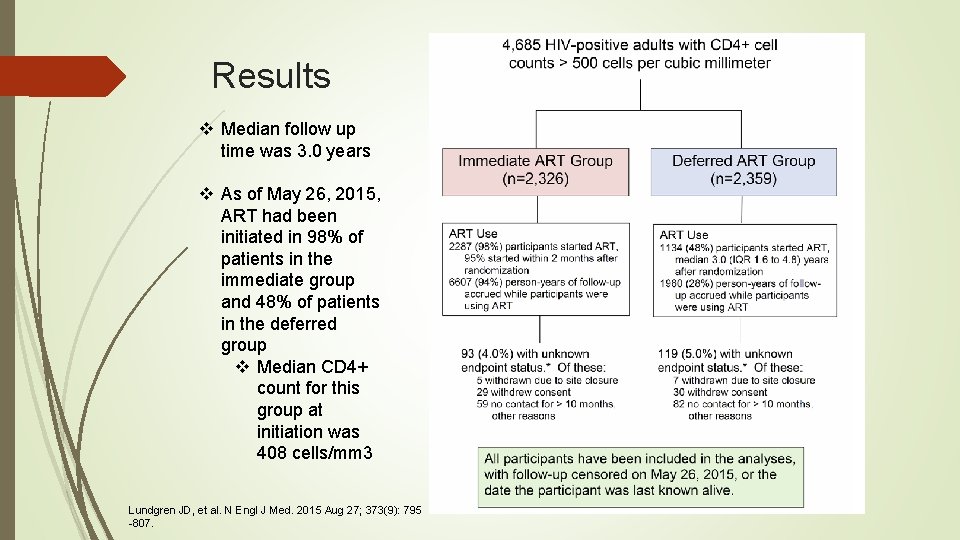
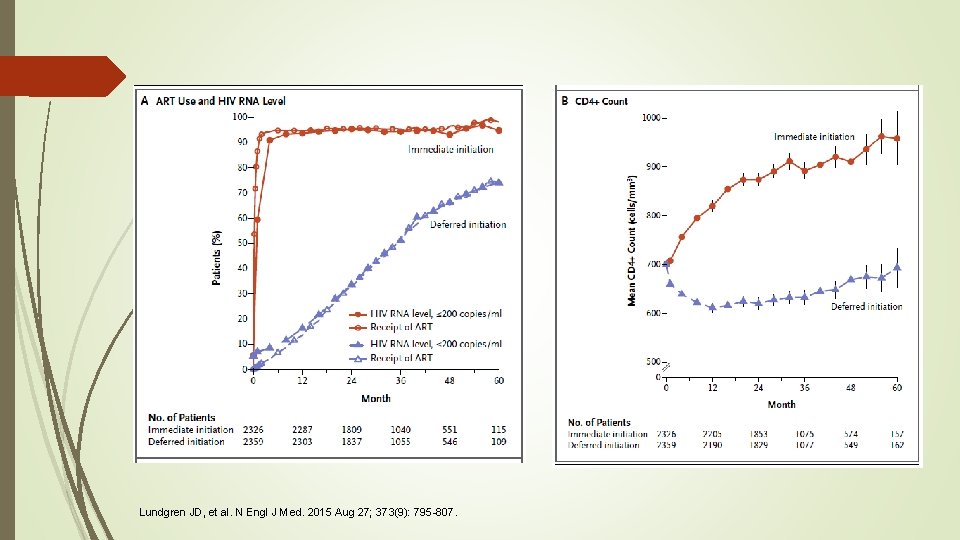
Results v Median follow up time was 3. 0 years v As of May 26, 2015, ART had been initiated in 98% of patients in the immediate group and 48% of patients in the deferred group v Median CD 4+ count for this group at initiation was 408 cells/mm 3 Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Results Patients received ART for 94% of total follow-up time accrued in the immediate group and 28% in the deferred group The percentage of patients who had an HIV RNA level of <200 copies/m. L during follow-up mirrored the percentage who were receiving ART Levels were lower in the immediate group than in the deferred group at the time of initiation The percentages of patients in full viral suppression by 12 months after initiation were similar (98% vs. 97%) The average CD 4+ counts increased markedly during the 1 st year after randomization in the immediate group. However, in the deferred group, average CD 4+ counts decreased during the 1 st year and then stabilized and subsequently increased slightly as more patients started ART Average CD 4+ count was 194 cells/mm 3 higher in immediate group than in deferred group during follow up period Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

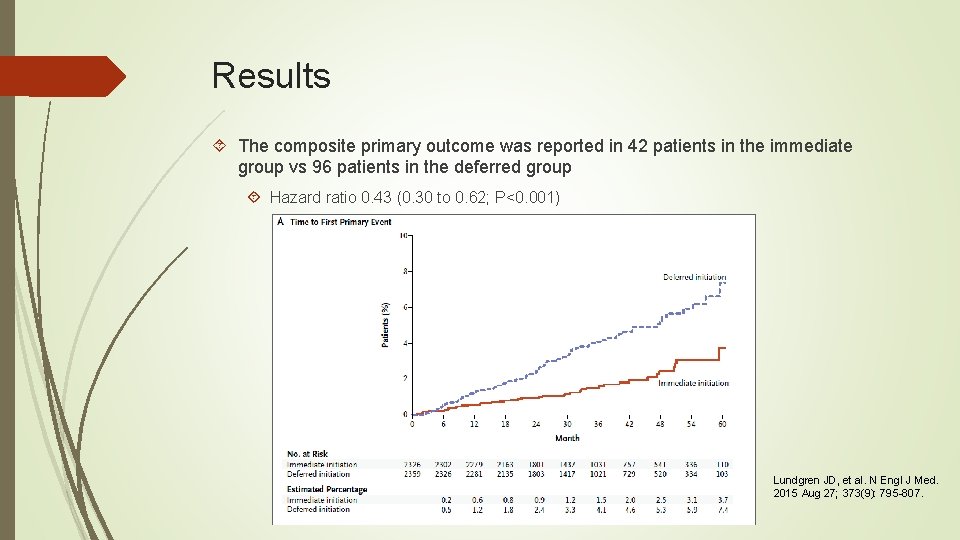
Results The composite primary outcome was reported in 42 patients in the immediate group vs 96 patients in the deferred group Hazard ratio 0. 43 (0. 30 to 0. 62; P<0. 001) Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Results Among the individual events included in the composite primary outcome, the 3 most common events in the immediate and deferred groups were CV disease(29% and 19%), non-AIDS defining cancer (21% and 19%) and tuberculosis (14% and 20%) Of the 42 primary endpoints, only 4(10%) occurred before initiation of ART in the immediate group as compared with 68 of 96 (71%) in the deferred group Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

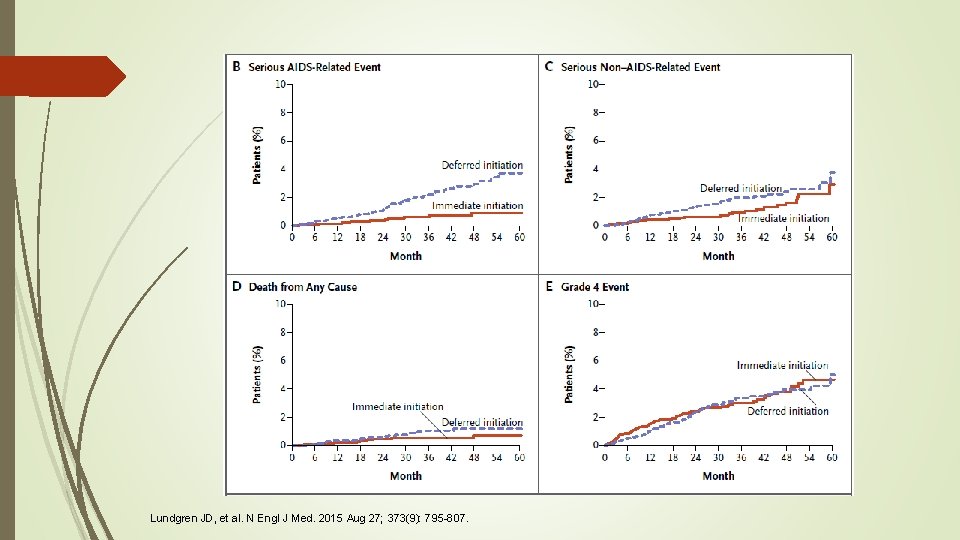
Tolerability & Safety: ADE Results Serious AIDS related event (3 most common were TB, Kaposi’s sarcoma and malignant lymphomas) Hazard ratio 0. 28 (0. 15 to 0. 50; P<0. 001) Serious non AIDS related event (2 most common were non-AIDS cancer and CV disease) Hazard ratio 0. 61 (0. 38 to 0. 97; P = 0. 04) Death from any cause Hazard ratio 0. 58 (0. 28 to 1. 17; P=0. 13) Of the 33 deaths, 20 (61%) were attributable to causes other than AIDS, CV disease, renal disease, liver disease or cancer Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

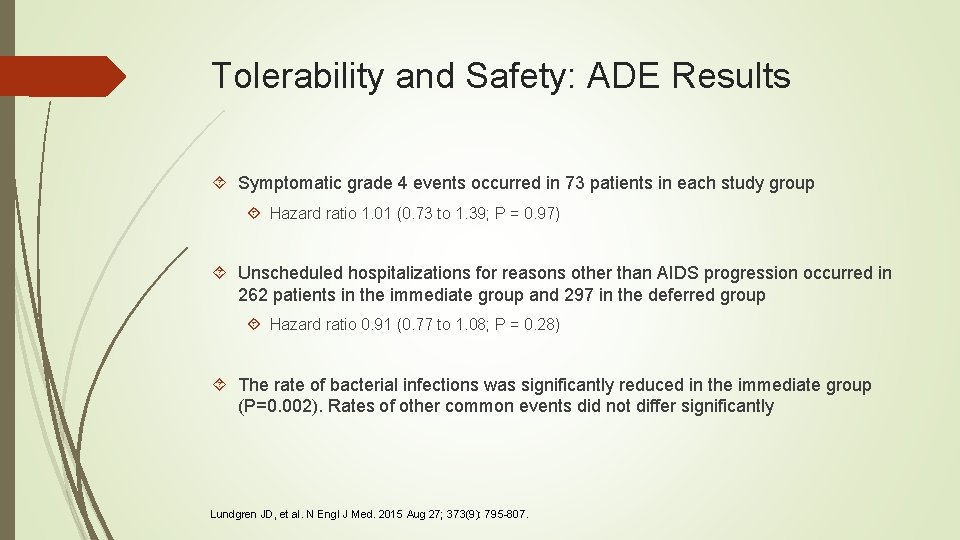
Tolerability and Safety: ADE Results Symptomatic grade 4 events occurred in 73 patients in each study group Hazard ratio 1. 01 (0. 73 to 1. 39; P = 0. 97) Unscheduled hospitalizations for reasons other than AIDS progression occurred in 262 patients in the immediate group and 297 in the deferred group Hazard ratio 0. 91 (0. 77 to 1. 08; P = 0. 28) The rate of bacterial infections was significantly reduced in the immediate group (P=0. 002). Rates of other common events did not differ significantly Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Tolerability and Safety: ADE Results For a composite end point that included grade 4 events, unscheduled hospitalizations and the primary end point as an overall measure of clinical benefit, there were 295 patients in the immediate group and 355 in the deferred group who had an event Hazard ratio 0. 82 (0. 71 to 0. 96; P = 0. 01) 16 suspected, unexpected serious ADR were reported among patients receiving ART Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

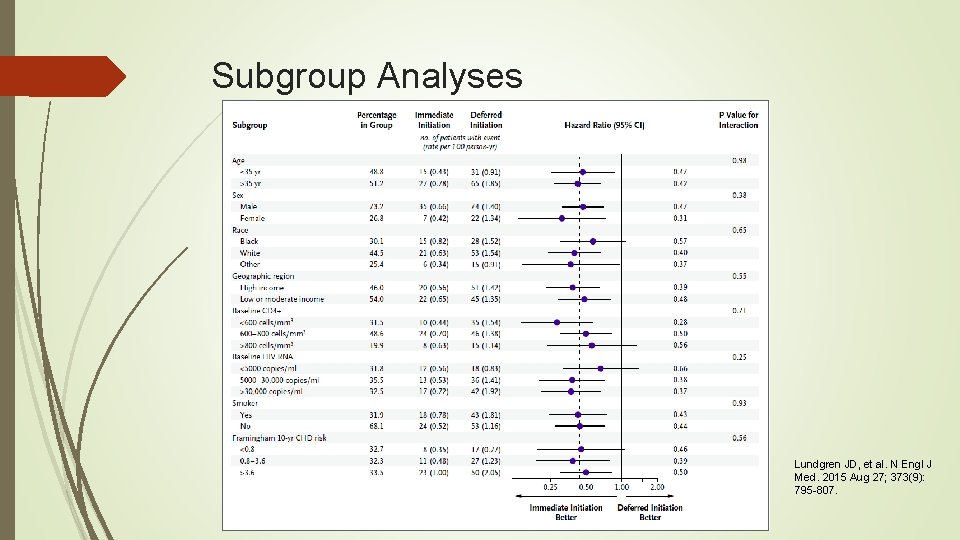
Results The majority of primary events occurred when the CD 4+ count was greater than 500 cells/mm 3 in 37 of 42 patients (88%) in the immediate group and in 57 of the 96 (59%) in the deferred group Latest CD 4+ counts were <350 cells/mm 3 for only 4% of follow-up time in the deferred group Risk of developing serious AIDS, serious non-AIDS, or death was reduced by 57% among those in the immediate group, compared to those in the deferred group. This reduction was seen regardless of age, sex, baseline CD 4+ cell counts, geographic region or country income level. Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Subgroup Analyses Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Discussion Strengths: Large sample size in many countries Appropriate patient population Intention-to-treat Funding by National Institute of Allergy and Infectious Diseases (NIAID) Success in answering primary question/assessing primary objective

Discussion Limitations: Lower than anticipated rate of non-AIDS related serious events combined with early termination of deferred-ART arm resulted in low statistical power to quantify benefit This limits our understanding of the effects of immediate therapy on the risk of individual serious non. AIDS conditions Mean follow-up of 3 years is a short period to assess risks and benefits of chronic ART (lifelong treatment) Lack of analysis on long-term ART Although it was funded by NIAID, ART medications were donated by Abb. Vie, Bristol-Myers Squibb, Gilead Sciences, Glaxo. Smith. Kline/Vii. V Healthcare, Janssen Scientific Affairs, and Merck Lack of blinding No set medication regimen for the study protocol Side effects seen could be location dependent

Discussion Appropriate Statistics Intention-to-treat Appropriate Kaplan Meier Appropriate Cox-proportional hazards Appropriate

Author’s Conclusions There is a significant benefit in immediate initiation of ART in patients with HIB infection regardless of CD 4+ count. These results align benefits for HIV+ individuals with the public health benefit in reducing risk of viral transmission with initiation of ART Health systems need to improve programs and be more accessible for patients to help in the diagnosis of HIV diagnosis and ensuring proper care for patients Lundgren JD, et al. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.

Learner’s Conclusions Initiation of antiretroviral therapy is beneficial regardless of CD 4 count; however patient specific factors should still be taken into consideration. Immediate initiation of ART is beneficial for not only the HIV-infected individual but for the society as a whole Serious adverse events seen in this study may have been influenced by location/geography. However, all serious adverse events should be monitored regardless of location

Impact on Current Clinical Practice Based on the START and TEMPRANO findings, the Panel on Antiretroviral Guidelines for Adults and Adolescents (the Panel) has increased the strength and evidence rating for the recommendation on initiating ART to AI for all HIV-infected patients, regardless of CD 4 count. Gulick R et al. J Med Pract. 2016; 5(2): 1 -277.

Further Studies Needed Study with a longer follow up period to assess risks vs. benefit of long-term (i. e. lifelong) ART Further follow up on the benefit of immediate ART with the risk of developing serious non AIDS condition(s) Studies with a set medication regimen/protocol so every patient receives the same medication classes to assess risk vs benefit Further studies in specific locations to assess risk for potential adverse events

Questions?

References Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009; 360(18): 1815 -26. HIV-CAUSAL Collaboration, Cain LE, Logan R, Robins JM, Sterne JA, Sabin C, et al. When to initiate combined antiretroviral therapy to reduce mortality and AIDSdefining illness in HIV-infected persons in developed countries: an observational study. Ann Intern Med. 2011 Apr 19; 154(8): 509 -15. Gulick R, Hirsch M, Clifford H, et al. Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Afr J Med Pract. 2016; 5(2): 1 -277. Lundgren JD, Babiker AG, Gordin F, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015 Aug 27; 373(9): 795 -807.
 Antiretroviral
Antiretroviral Antiretroviral
Antiretroviral Asymptomatic hyperglycemia
Asymptomatic hyperglycemia Asymptomatic bacteriuria
Asymptomatic bacteriuria Asymptomatic bacteriuria
Asymptomatic bacteriuria Asymptomatic bacteriuria
Asymptomatic bacteriuria Bacteriuria
Bacteriuria Asymptomatic bacteriuria
Asymptomatic bacteriuria What causes a miscarriage
What causes a miscarriage Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Bioness integrated therapy system price
Bioness integrated therapy system price Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Early cpr and early defibrillation can: *
Early cpr and early defibrillation can: * Sensibilisation et initiation à la cybersécurité
Sensibilisation et initiation à la cybersécurité A quoi sert la qualité
A quoi sert la qualité Site initiation visit in clinical trials ppt
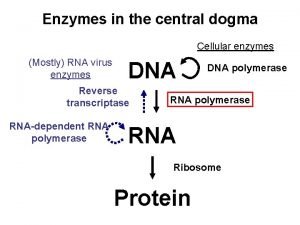
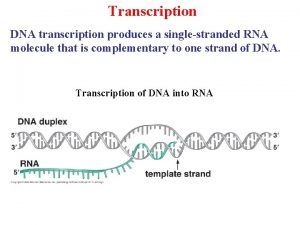
Site initiation visit in clinical trials ppt Central dogma
Central dogma Separation initiation return
Separation initiation return Jeanine
Jeanine Project initiation document
Project initiation document Stepwise topic shift
Stepwise topic shift Knapp intensifying stage
Knapp intensifying stage L'initiation
L'initiation Tahap inisiasi proyek
Tahap inisiasi proyek Project identification and selection
Project identification and selection Clozapine initiation chart
Clozapine initiation chart Separation initiation return
Separation initiation return The fall archetype
The fall archetype Importance of project initiation
Importance of project initiation Initiation promotion progression
Initiation promotion progression Initiation à la démonstration 5ème
Initiation à la démonstration 5ème Initiation and maintenance of callus culture
Initiation and maintenance of callus culture Define home visit
Define home visit Separation initiation return
Separation initiation return Rna polymerase
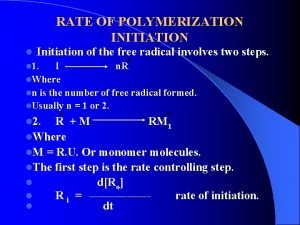
Rna polymerase Energy amplifier initiation
Energy amplifier initiation Site initiation visit
Site initiation visit