In Your Own Words Write a welldeveloped paragraph



























- Slides: 68

In Your Own Words. . . Write a well-developed paragraph explaining solutions. Use the key terms: solute, solvent, solution, solvation, soluble, insoluble, and Tyndall effect. You will turn in this assignment. Write your name, period number, today’s date, and page #U 8 -3 on your paper.

Chapter 15 Solutions

Solutions � Homogeneous mixtures � Gases, liquids, or solids Gas in a gas: air Gas in a liquid: sodas Liquid in a liquid: alcohol and water Solid in a solid: alloys Solid in a liquid (most common) � Two components Solvent: dissolves other materials; present in greater amount Water is universal solvent Solute: being dissolved; present in lesser amount May have more than one solute (e. g. , ocean water)

Solutions �Concentration describes the amount of solute dissolved in solution �Dilute solutions contain a small amount of solute �Concentrated solutions contain a large amount of solute

Solutions Quiz homogeneous mixture of two or �A solution is a ______ more substances. �Homogeneous means that you cannot parts distinguish the ____, and the mixture is in phase or state one _______. the tea �In sweet tea, the solvent is _____and the sugar solute is ______. Water �____ is the universal solvent. air �An example of a gaseous solution is ______. solids �An alloy is a solution made of ____. An brass or steel example of an alloy is ______. water �In aqueous solutions, ____ is the solvent.

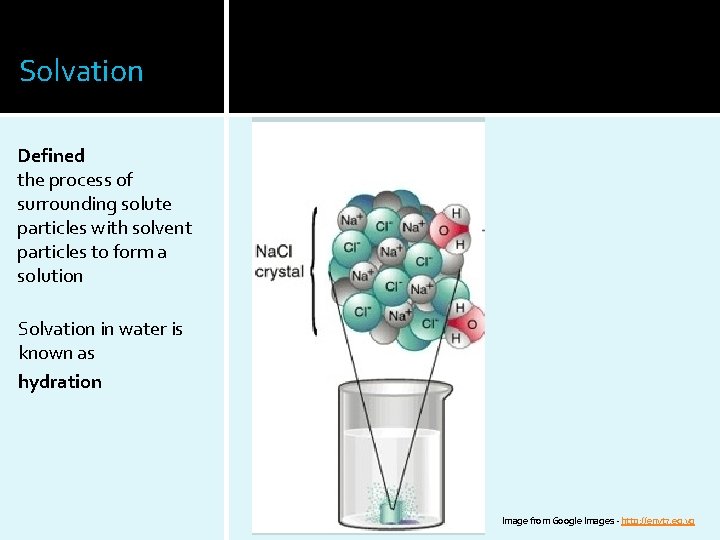
Solvation Defined the process of surrounding solute particles with solvent particles to form a solution Solvation in water is known as hydration Image from Google Images - http: //envt 7. eg. vg

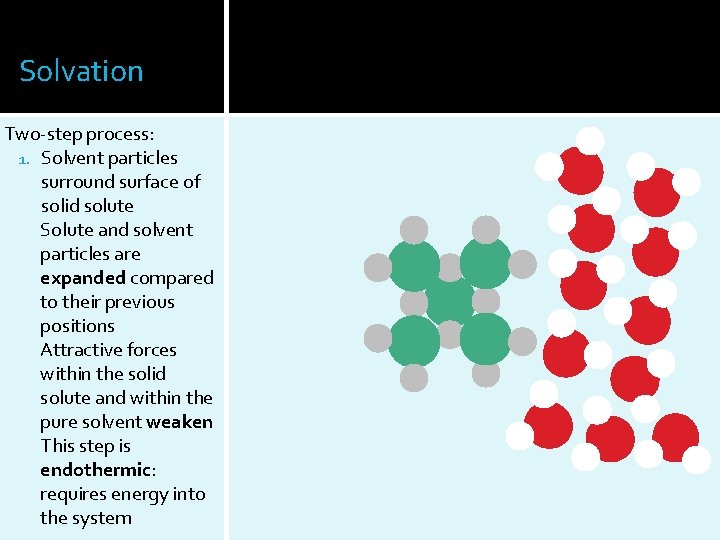
Solvation Two-step process: 1. Solvent particles surround surface of solid solute Solute and solvent particles are expanded compared to their previous positions Attractive forces within the solid solute and within the pure solvent weaken This step is endothermic: requires energy into the system

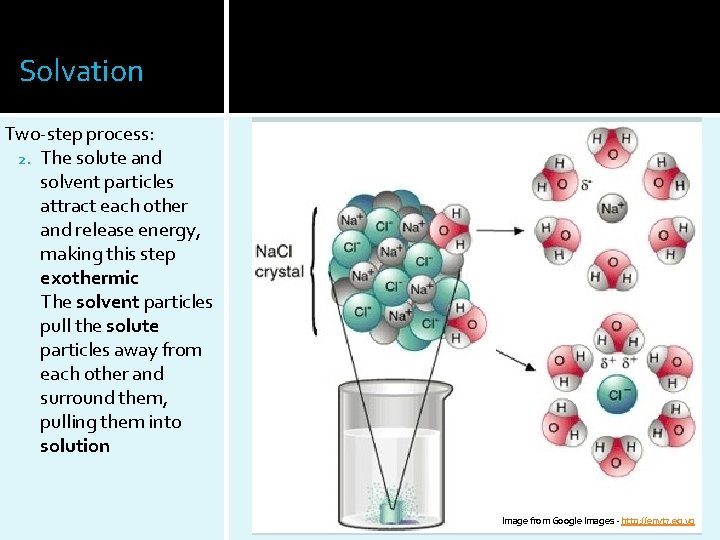
Solvation Two-step process: 2. The solute and solvent particles attract each other and release energy, making this step exothermic The solvent particles pull the solute particles away from each other and surround them, pulling them into solution Image from Google Images - http: //envt 7. eg. vg

Solvation Heat of solution: The overall energy change when a solution is formed May be endothermic or exothermic Image from Google Images - http: //envt 7. eg. vg

Factors Affecting Solvation � Increasing the number of collisions between solute and solvent increases rate of dissolving � Agitation Stirring or shaking increases number of collisions � Surface Area Breaking or grinding into smaller pieces increases surface area, and more collisions can occur � Temperature Measure of kinetic energy (movement of particles) Higher temperature → more collisions → more solid dissolves Lower temperature → more gas dissolves � Pressure Does not affect the solubility of solids or liquids Increased pressure increases solubility of gas

Solvation Writing (Homework due 02/15) In a well-developed writing, explain the process of solvation. Define what is meant by solvation. Discuss the two steps and their energy requirements. Explain the factors that affect solvation. Include the terms: solvation, hydration, solute, solvent, exothermic, endothermic, heat of solution, collisions, agitation, surface area, temperature, pressure.

Solvation and Polarity � Polar molecules are dipoles Partially positive end Partially negative end � Polar solutes tend to dissolve in polar solvents � Ionic compounds (Na. Cl) and polar molecular compounds (C 12 H 22 O 11) dissolve in water (polar) � Nonpolar solutes tend to dissolve in nonpolar solvents � Octane and benzene (nonpolar liquids) will dissolve in each other � When mixed, polar and nonpolar substances form suspensions – not solutions � General rule known as like dissolves like

GO-GO-MO Set up your GO-GO-MO Table with the following column headings. Topic My Info About Topic A Friend’s Info (GO) A Different Friend’s Info (MO) We will add to this table during this unit.

GO-GO-MO Set up your GO-GO-MO Table with the following column headings. My Info About Topic A Friend’s Info (GO) Topic A Different Friend’s Info (MO) 1. Homogeneous mixtures We will add to this table during this unit.

GO-GO-MO Set up your GO-GO-MO Table with the following column headings. My Info About Topic A Friend’s Info (GO) Topic A Different Friend’s Info (MO) 1. Homogeneous mixtures 2. Solvation process We will add to this table during this unit.

GO-GO-MO Set up your GO-GO-MO Table with the following column headings. My Info About Topic A Friend’s Info (GO) Topic A Different Friend’s Info (MO) 1. Homogeneous mixtures 2. Solvation process 3. Factors affecting solvation We will add to this table during this unit.

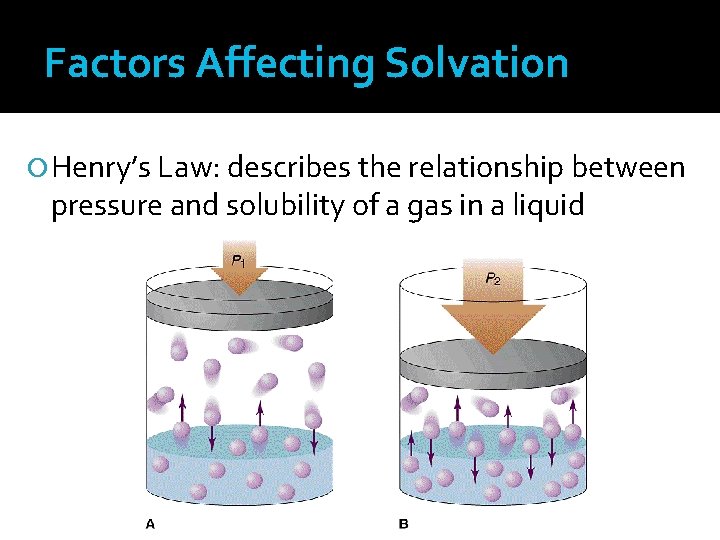
Factors Affecting Solvation Henry’s Law: describes the relationship between pressure and solubility of a gas in a liquid

Applying Henry’s Law Sodas are prepared by dissolving a gas solute CO 2 in a _____ ( usually _____) solvent liquid The carbon dioxide gas is most soluble when the drink is at ____ low temperature and high pressure) unopened (____ Once opened, the pressure _____ decreases The gas _______ leaves the solution

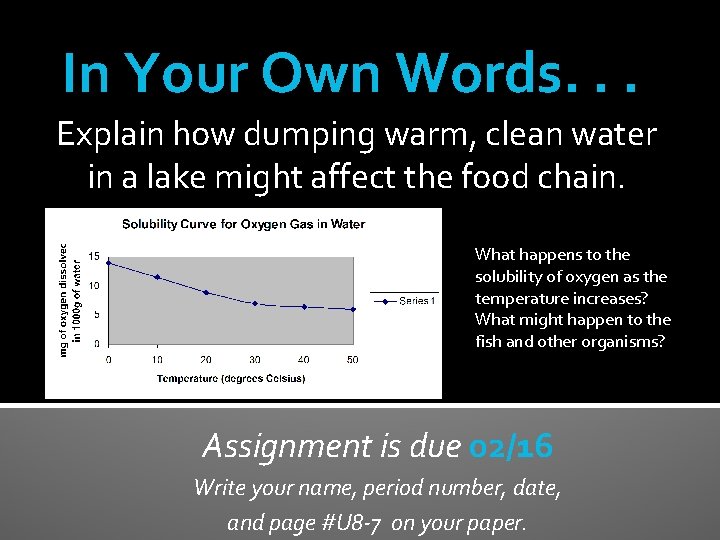
In Your Own Words. . . Explain how dumping warm, clean water in a lake might affect the food chain. What happens to the solubility of oxygen as the temperature increases? What might happen to the fish and other organisms? Assignment is due 02/16 Write your name, period number, date, and page #U 8 -7 on your paper.

Key Terms �Soluble: Describes a solute that will dissolve in a given solvent �Miscible: Describes two liquids that will dissolve in each other �Insoluble: Describes a solute that will not dissolve in a given solvent �Immiscible: Describes two liquids that will not dissolve in each other

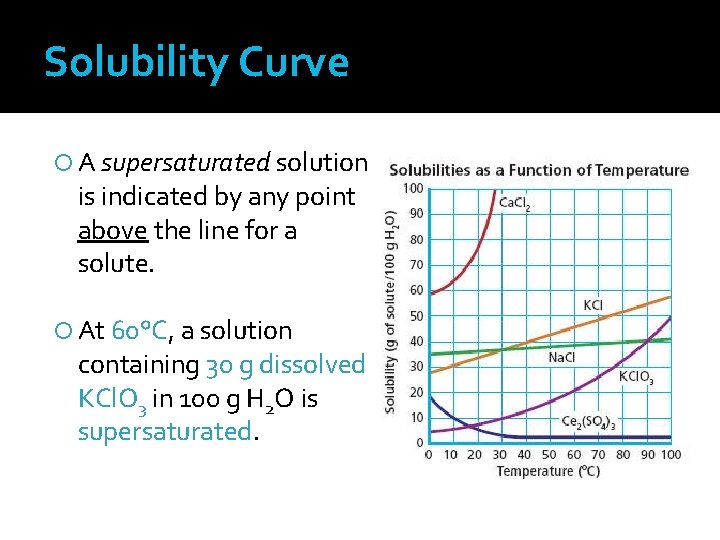
Solubility � A measure of the amount of solute that dissolves in a given amount of solvent at a specific temperature and pressure � Saturated solutions Contain the maximum amount of dissolved solute for a given amount of solvent at a specific temperature and pressure Have established chemical equilibrium � Unsaturated solutions Contain less than the maximum amount of dissolved solute for a given amount of solvent at a specific temperature and pressure Can dissolve more solute � Supersaturated solutions Contain more dissolved solute than a saturated solution at the same temperature and pressure Will not dissolve additional solute (will fall to bottom of beaker)

Solubility �Formation of supersaturated solutions: Create saturated solution at high temperature Slowly cool the solution to maintain the excess solute at the lower temperature At the lower temperature, the solution is considered supersaturated �Supersaturated solutions are unstable Recrystallization occurs when the container is disturbed or agitated in any way or if a tiny amount of solute (seed crystal) is added to the container Solution returns to the point of saturation

Solutions Quiz � A substance that dissolves in another is considered soluble ____. insoluble � Sand water are _____. miscible � Two liquids that are soluble are called _____. Hydration is solvation with water. � _____ � Factors that increase solvation for solid solutes agitation __________, increasing surface area and include: ____, increasing temperature __________. � Like dissolves like means that polar substances polar molecules ionic compounds and _______. dissolve _______ suspension instead of a solution � Oil and water form a _____ nonpolar and water is ____. polar because oil is ____

Solutions Quiz Solubility refers to the amount of solute that will � _____ dissolve in a given amount of solvent at a specific temperature and pressure. A saturated solution contains the maximum � ______ amount of dissolved solute at a given temperature and pressure. an unsaturated � More solute can be dissolved in ________ solution. � Making a supersaturated solution involves creating a saturated solution at a ____temperature high _____ and cooling it to the desired temperature. slowly _____ seed crystal to a supersaturated solution � Adding a ______ saturation will return it to the point of ______.

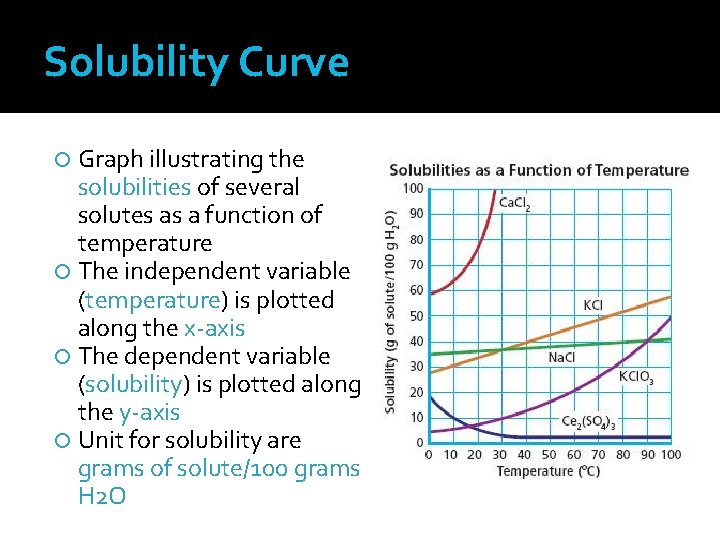
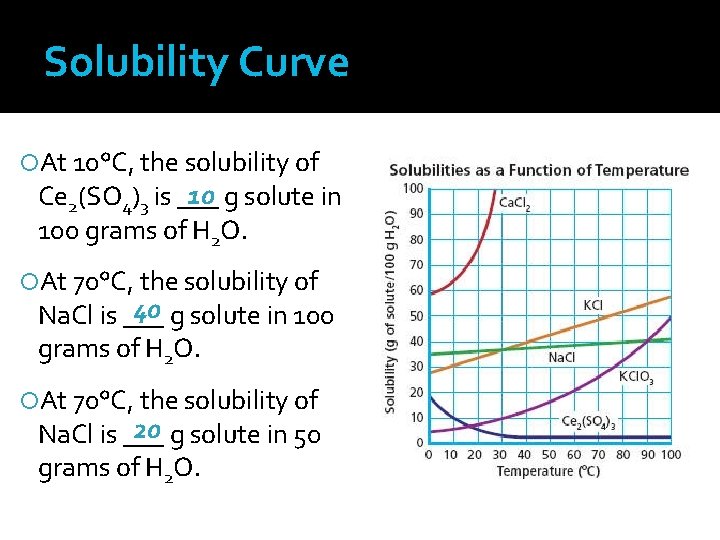
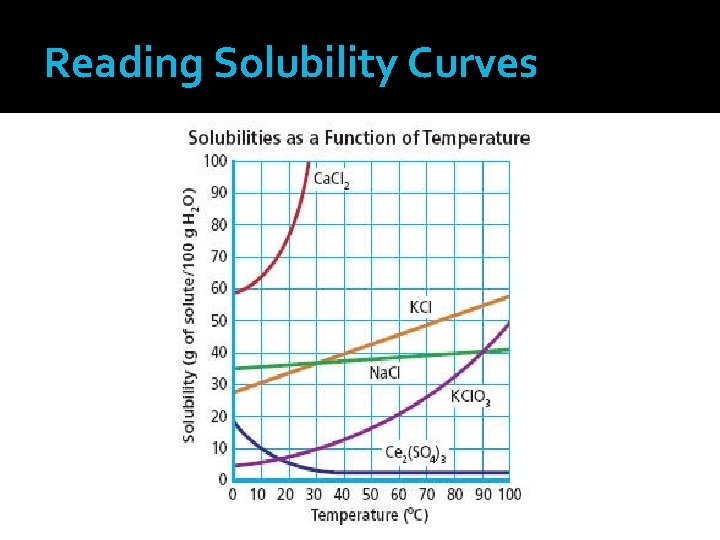
Solubility Curve Graph illustrating the solubilities of several solutes as a function of temperature The independent variable (temperature) is plotted along the x-axis The dependent variable (solubility) is plotted along the y-axis Unit for solubility are grams of solute/100 grams H 2 O

Creating a Supersaturated Solution In a well-developed paragraph, explain how to make a supersaturated solution of potassium chloride and water. Turn in the assignment before you leave class. Write your name, period number, date, and page #U 8 -8 on your paper.

Solutions Quiz polar solvent. Toluene is a �Water is a _____ nonpolar liquid. These two liquids would be immiscible described as _____. ionic compound �Potassium chloride is an _____ polar molecule. and ammonia is a _____ nonpolar molecule. �Hexane is a _______ �Will the following pairs form a solution? water and potassium chloride water and ammonia toluene and ammonia hexane and toluene yes no yes

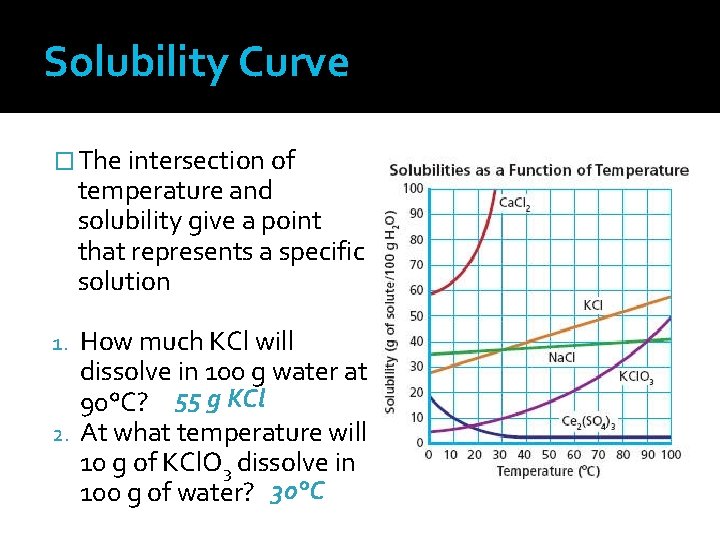
Solubility Curve � The intersection of temperature and solubility give a point that represents a specific solution How much KCl will dissolve in 100 g water at 90°C? 55 g KCl 2. At what temperature will 10 g of KCl. O 3 dissolve in 100 g of water? 30°C 1.

Solubility Curve At 10°C, the solubility of 10 g solute in Ce 2(SO 4)3 is ___ 100 grams of H 2 O. At 70°C, the solubility of 40 g solute in 100 Na. Cl is ___ grams of H 2 O. At 70°C, the solubility of 20 g solute in 50 Na. Cl is ___ grams of H 2 O.

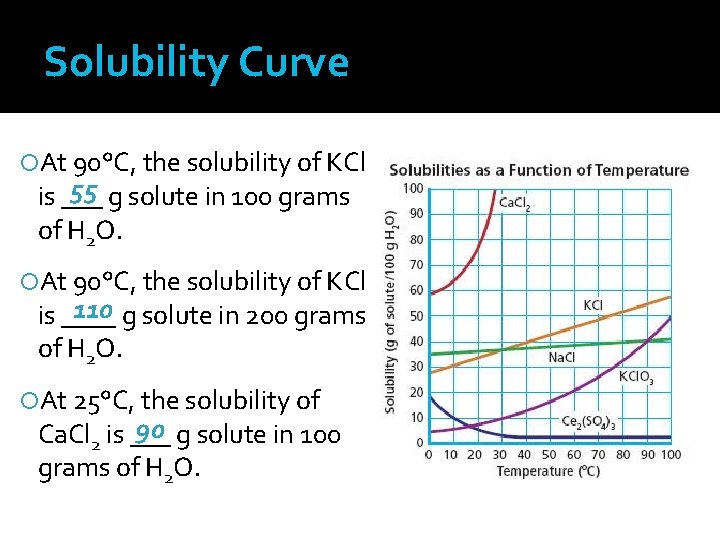
Solubility Curve At 90°C, the solubility of KCl 55 g solute in 100 grams is ___ of H 2 O. At 90°C, the solubility of KCl 110 g solute in 200 grams is ____ of H 2 O. At 25°C, the solubility of 90 g solute in 100 Ca. Cl 2 is ___ grams of H 2 O.

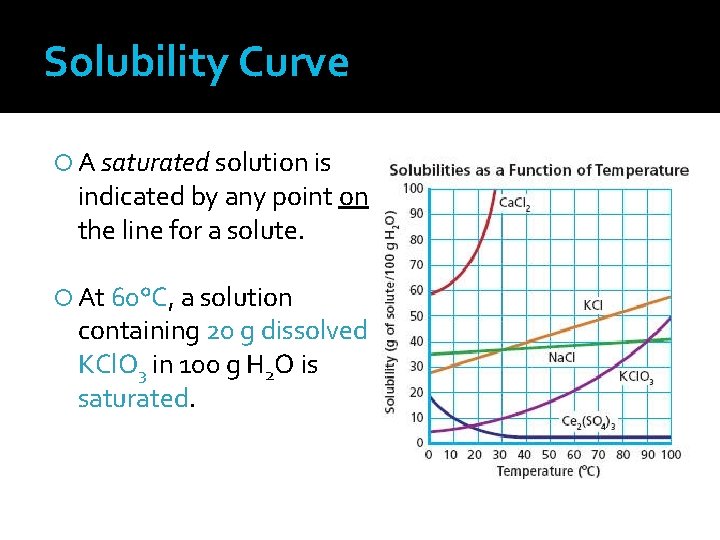
Solubility Curve A saturated solution is indicated by any point on the line for a solute. At 60°C, a solution containing 20 g dissolved KCl. O 3 in 100 g H 2 O is saturated.

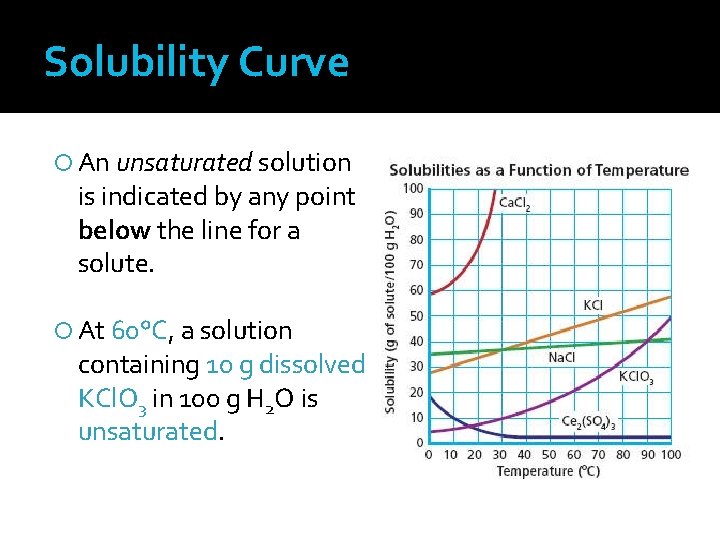
Solubility Curve An unsaturated solution is indicated by any point below the line for a solute. At 60°C, a solution containing 10 g dissolved KCl. O 3 in 100 g H 2 O is unsaturated.

Solubility Curve A supersaturated solution is indicated by any point above the line for a solute. At 60°C, a solution containing 30 g dissolved KCl. O 3 in 100 g H 2 O is supersaturated.

Reading Solubility Curves

Solubility Curve Quiz saturated solutions are � On a solubility curve, ______ represented by points on the curve for a given solute. � Solution indicated by points below the curve for a unsaturated solute are classified as ______. � Solutions created at high temperatures and then slowly cooled to the desired temperature are known supersaturated and are indicated by points as ________ above the curve for a solute. _____ grams � The units for solubility are _____ of solute per 100 grams of water __________. � Adding a seed crystal to a supersaturated solution precipitate out of causes the excess solute to ______ solution.

Solubility Curves Practice At what temperature and gram amount could you make a perfectly saturated solution of calcium chloride? 4°C, 60 g 25°C, 90 g What temperature and gram amount would you need to make an unsaturated solution of cerium sulfate? 0°C, 10 g 10°C, 5 g

A solution of potassium chlorate is made using 40 grams at 90°C, and then slowly cooled to 60°C. At the lower temperature, what kind of a solution is this? supersaturated If an additional crystal of potassium chlorate is added to the above solution, what will happen? The excess solute will precipitate out of solution. How much precipitate will be formed? 20 g KCl. O 3

Solubility Curves Practice What gram amount of sodium chloride would you need to make a perfectly saturated solution at 30°C? 37. 5 g Na. Cl If you place 75 grams of calcium chloride in 100 g of water at 10°C, what kind of a solution do you have? saturated To what temperature would you have to heat the solution in order to dissolve all remaining mass? 20°C

Solubility Curves Practice What is the solubility of potassium chlorate in 100 grams of water at 100°C? 48 g KCl. O 3 What is the solubility of potassium chloride in 100 grams of water at 50°C? 42. 5 g KCl What is the solubility of sodium chloride in 100 grams of water at 90°C? 40 g Na. Cl

Solubility Curve Practice What is the minimum temperature needed to dissolve 90 grams of calcium chloride in 100 grams of water? 25°C What is the minimum temperature needed to dissolve 35 grams of potassium chloride in 100 grams of water? 25°C

Solubility Curve Practice At what temperature do potassium chloride and sodium chloride have the same solubility in 100 grams of water? 30°C At what temperature do sodium chloride and potassium chlorate have the same solubility in 100 grams of water? 90°C

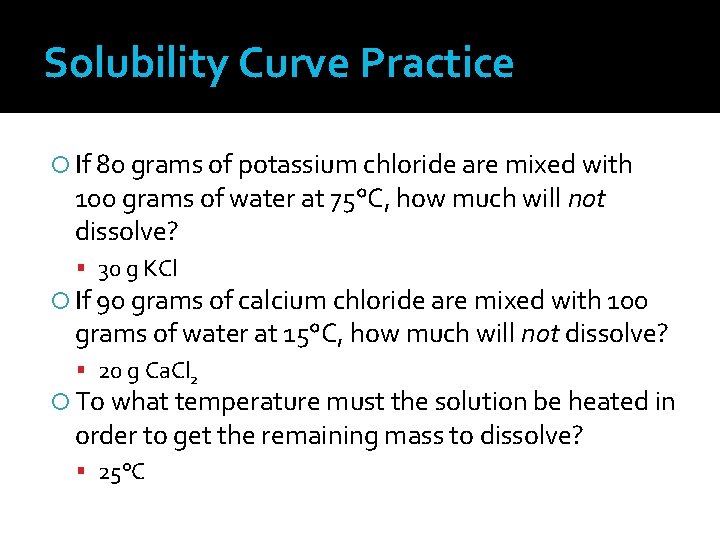
Solubility Curve Practice If 80 grams of potassium chloride are mixed with 100 grams of water at 75°C, how much will not dissolve? 30 g KCl If 90 grams of calcium chloride are mixed with 100 grams of water at 15°C, how much will not dissolve? 20 g Ca. Cl 2 To what temperature must the solution be heated in order to get the remaining mass to dissolve? 25°C

Solubility Curve Practice If 15 grams of potassium chlorate are added to 100 grams of water at 90°C, how much more must be added to saturate the solution? 25 g KCl. O 3 If 10 grams of sodium chloride are added to 100 grams of water at 80°C, how much more must be added to saturate the solution? 30 g Na. Cl

Solubility Curve Practice How much sodium chloride will dissolve in 100 grams of water at 10°C? 36 g Na. Cl How much sodium chloride will dissolve in 200 grams of water at 10°C? 72 g Na. Cl How much sodium chloride will dissolve in 50 grams of water at 10°C? 18 g Na. Cl

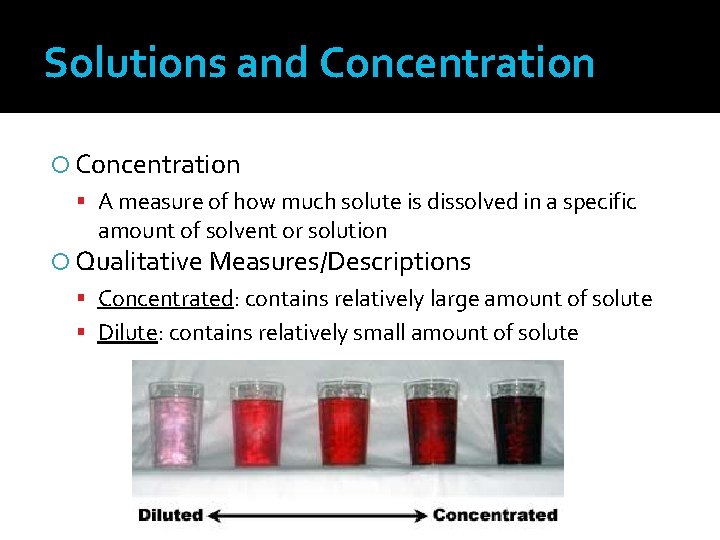
Solutions and Concentration A measure of how much solute is dissolved in a specific amount of solvent or solution Qualitative Measures/Descriptions Concentrated: contains relatively large amount of solute Dilute: contains relatively small amount of solute

Solutions and Concentration Quantitative Measures Use quantities expressed by numbers Formulas Percent Concentration by Mass Percent Concentration by Volume Molarity and Preparing Molar Solutions Dilutions Molality Henry’s Law

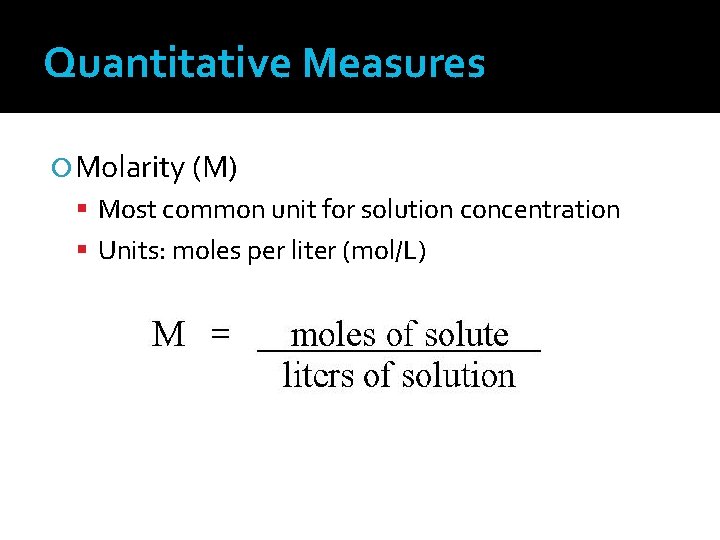
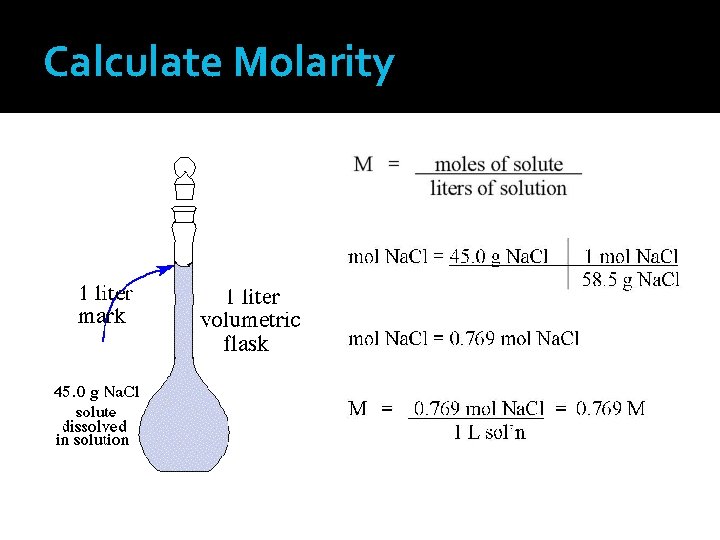
Quantitative Measures Molarity (M) Most common unit for solution concentration Units: moles per liter (mol/L)

Calculate Molarity

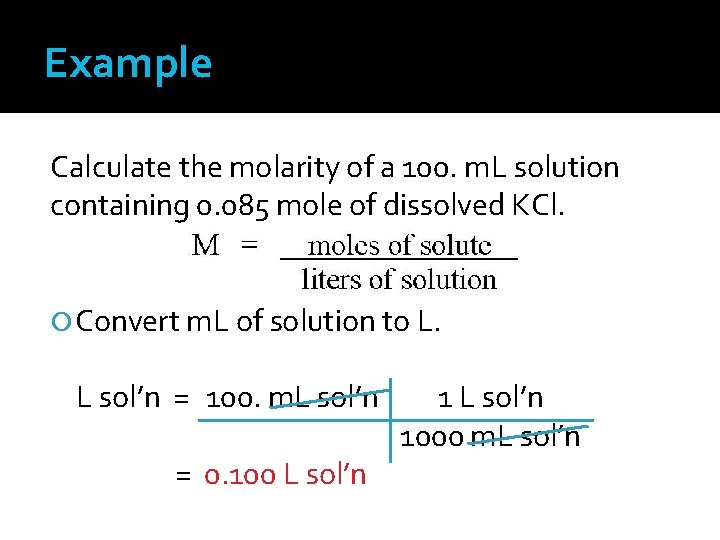
Example Calculate the molarity of a 100. m. L solution containing 0. 085 mole of dissolved KCl. Convert m. L of solution to L. L sol’n = 100. m. L sol’n = 0. 100 L sol’n 1000 m. L sol’n

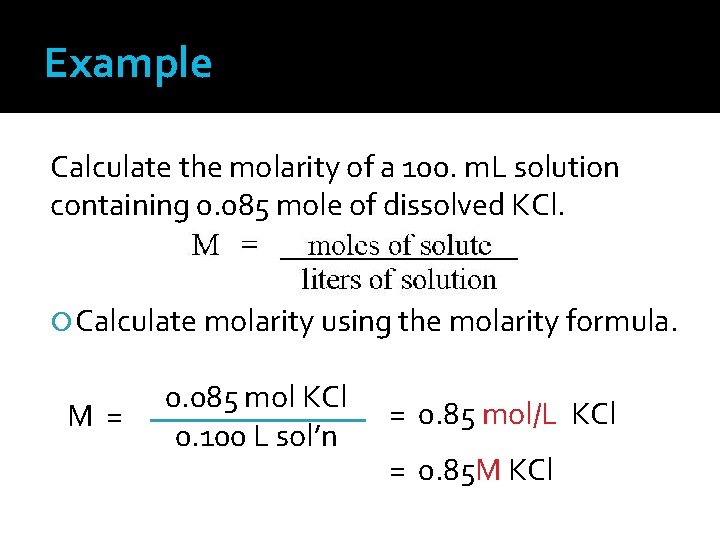
Example Calculate the molarity of a 100. m. L solution containing 0. 085 mole of dissolved KCl. Calculate molarity using the molarity formula. M= 0. 085 mol KCl 0. 100 L sol’n = 0. 85 mol/L KCl = 0. 85 M KCl

Molarity Practice Complete the five (5) molarity practice problems on page 6 of your Solutions Notes. Practice should be completed for tomorrow.

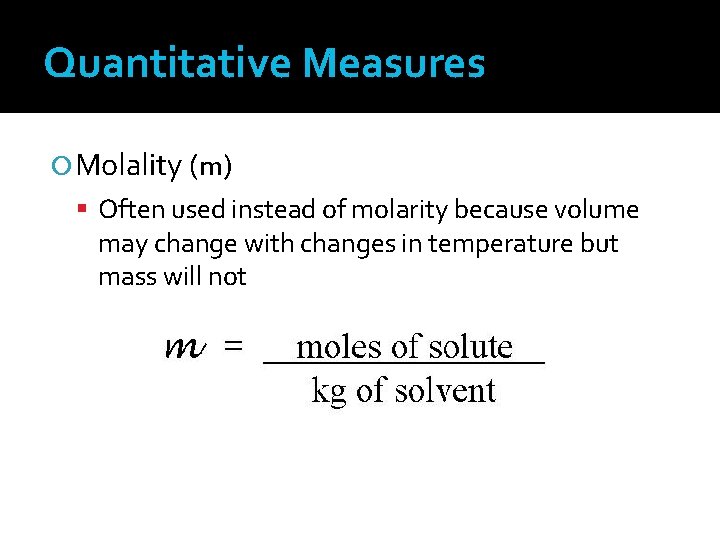
Quantitative Measures Molality (m) Often used instead of molarity because volume may change with changes in temperature but mass will not

Quantitative Measures Molality Example, p. 7 of Notes 0. 077 mol Na. Cl in 0. 1000 kg H 2 O creates a 0. 77 m solution Molality Practice, p. 7 of Notes 1. 01 mol KCl in 0. 095 kg H 2 O creates a 10. 6 m solution 0. 234 mol C 10 H 8 in 0. 500 kg toluene (C 7 H 8) creates a 0. 468 m solution

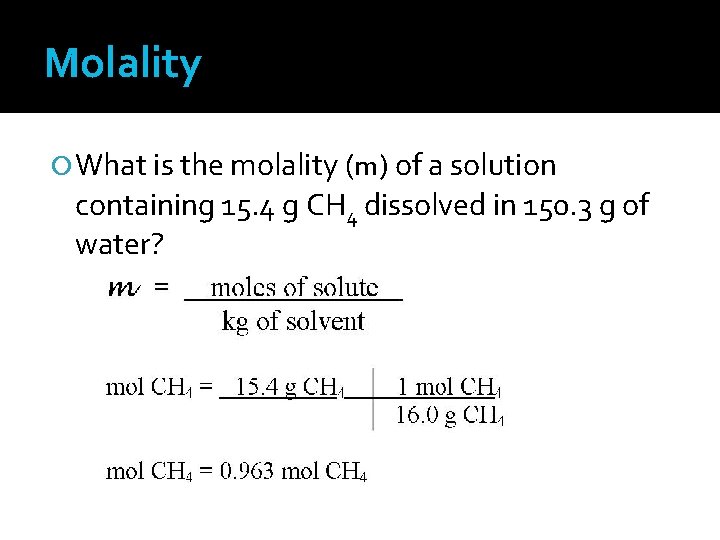
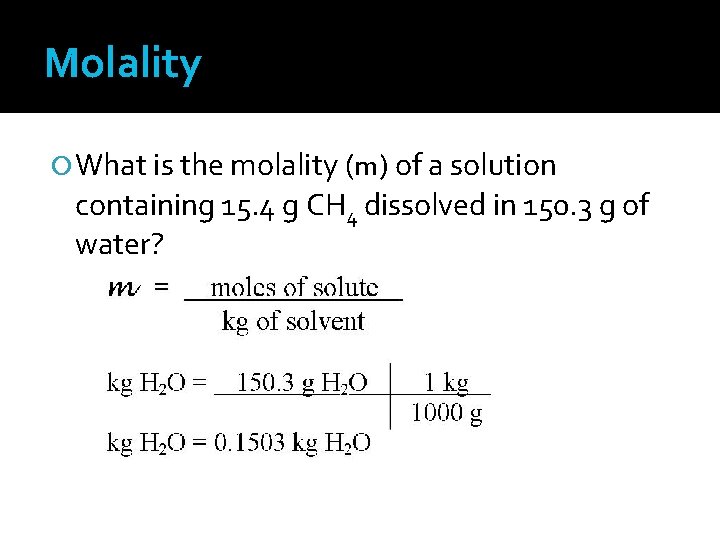
Molality What is the molality (m) of a solution containing 15. 4 g CH 4 dissolved in 150. 3 g of water?

Molality What is the molality (m) of a solution containing 15. 4 g CH 4 dissolved in 150. 3 g of water?

Molality What is the molality (m) of a solution containing 15. 4 g CH 4 dissolved in 150. 3 g of water?

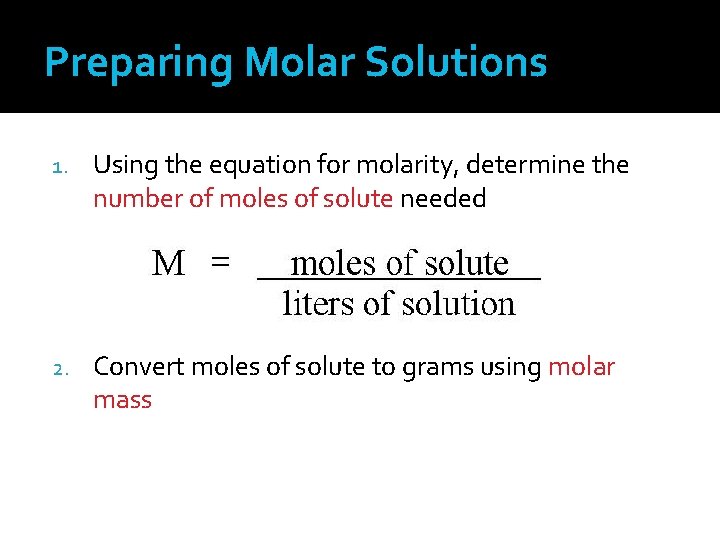
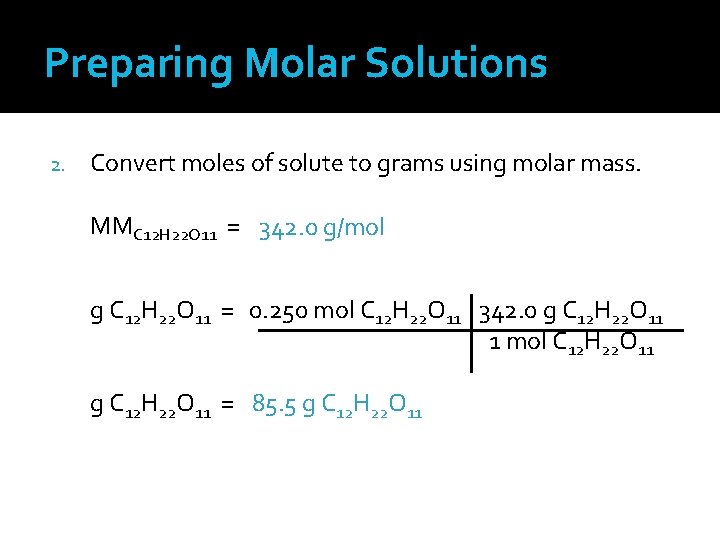
Preparing Molar Solutions 1. Using the equation for molarity, determine the number of moles of solute needed 2. Convert moles of solute to grams using molar mass

Preparing Molar Solutions Mass the amount of solute needed and add to a volumetric flask 4. Add solvent until the total volume reaches the necessary volume of solution 3. The volume of solution is different from the volume of solvent.

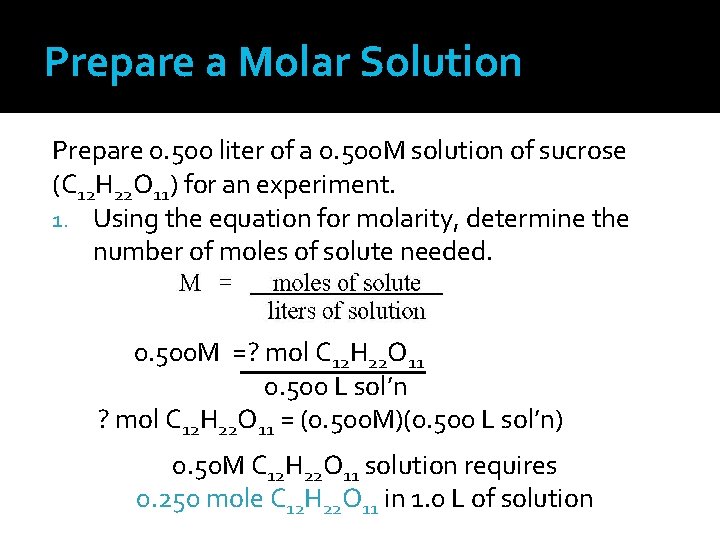
Prepare a Molar Solution Prepare 0. 500 liter of a 0. 500 M solution of sucrose (C 12 H 22 O 11) for an experiment. 1. Using the equation for molarity, determine the number of moles of solute needed. 0. 500 M =? mol C 12 H 22 O 11 0. 500 L sol’n ? mol C 12 H 22 O 11 = (0. 500 M)(0. 500 L sol’n) 0. 50 M C 12 H 22 O 11 solution requires 0. 250 mole C 12 H 22 O 11 in 1. 0 L of solution

Preparing Molar Solutions 2. Convert moles of solute to grams using molar mass. MMC 12 H 22 O 11 = 342. 0 g/mol g C 12 H 22 O 11 = 0. 250 mol C 12 H 22 O 11 342. 0 g C 12 H 22 O 11 1 mol C 12 H 22 O 11 g C 12 H 22 O 11 = 85. 5 g C 12 H 22 O 11

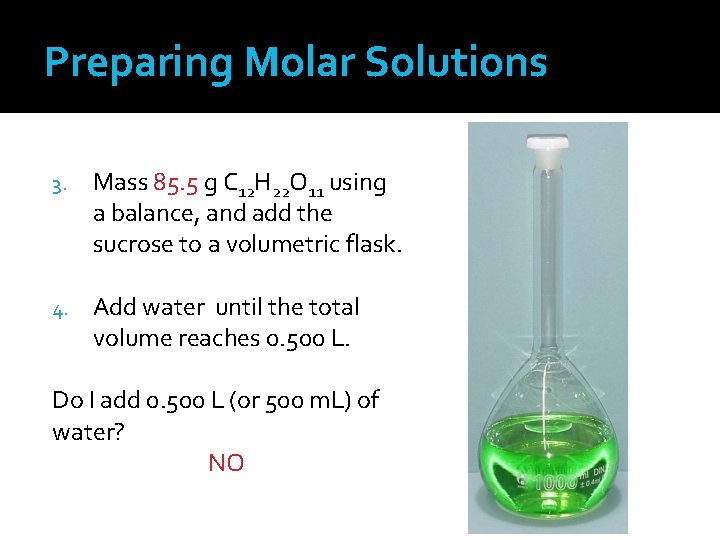
Preparing Molar Solutions 3. Mass 85. 5 g C 12 H 22 O 11 using a balance, and add the sucrose to a volumetric flask. 4. Add water until the total volume reaches 0. 500 L. Do I add 0. 500 L (or 500 m. L) of water? NO

Concentrate As a verb, the word concentrate means to “focus one’s attention or mental effort on a particular object or activity” (Google Search). In terms of solutions, however, concentrate means something very different.

Concentrate Orange juice is often purchased in the form of a concentrate. What does concentrate mean in this context?

Concentrate How does the orange juice get from the concentrate to the juice that we can drink?

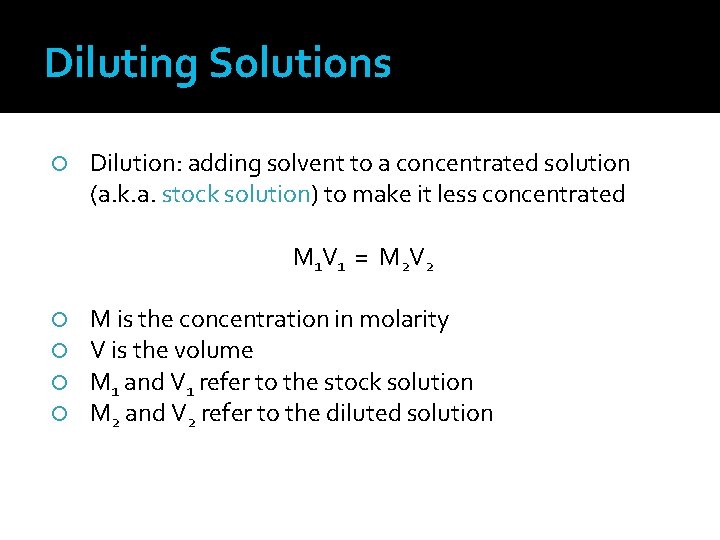
Diluting Solutions Dilution: adding solvent to a concentrated solution (a. k. a. stock solution) to make it less concentrated M 1 V 1 = M 2 V 2 M is the concentration in molarity V is the volume M 1 and V 1 refer to the stock solution M 2 and V 2 refer to the diluted solution

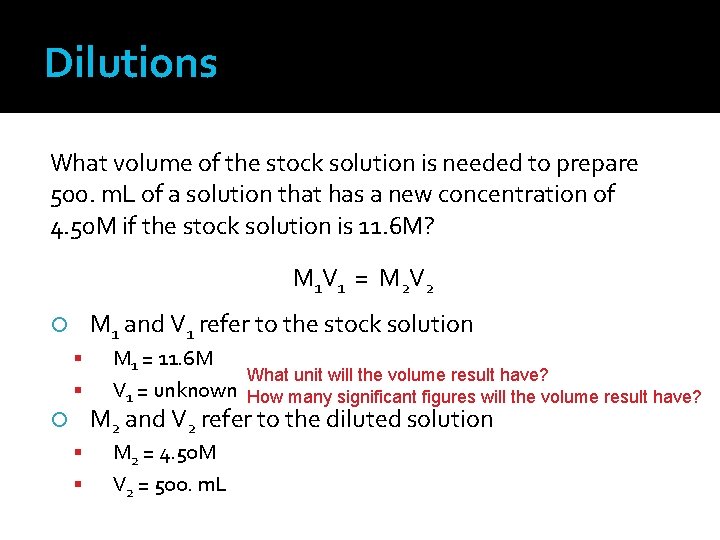
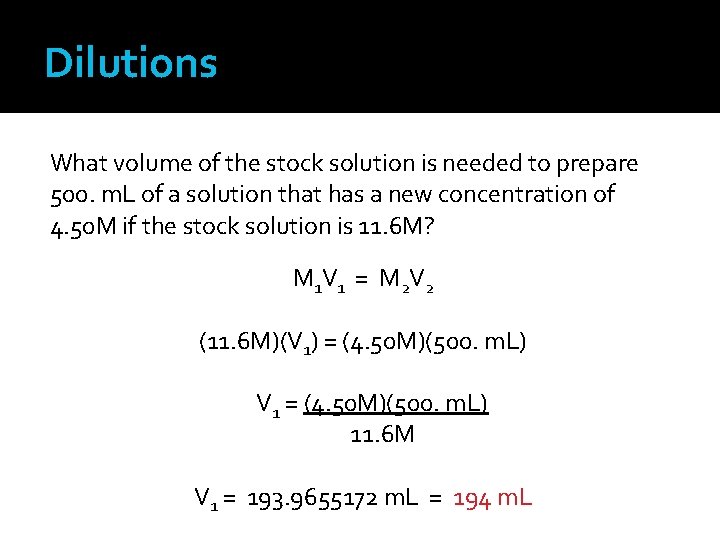
Dilutions What volume of the stock solution is needed to prepare 500. m. L of a solution that has a new concentration of 4. 50 M if the stock solution is 11. 6 M? M 1 V 1 = M 2 V 2 M 1 and V 1 refer to the stock solution M 1 = 11. 6 M What unit will the volume result have? V 1 = unknown How many significant figures will the volume result have? M 2 and V 2 refer to the diluted solution M 2 = 4. 50 M V 2 = 500. m. L

Dilutions What volume of the stock solution is needed to prepare 500. m. L of a solution that has a new concentration of 4. 50 M if the stock solution is 11. 6 M? M 1 V 1 = M 2 V 2 (11. 6 M)(V 1) = (4. 50 M)(500. m. L) V 1 = (4. 50 M)(500. m. L) 11. 6 M V 1 = 193. 9655172 m. L = 194 m. L

Practice: Molar Solutions and Dilutions Complete three (3) practice problems for Preparing Molar Solutions on p. 8 and the three (3) Dilutions practice problems on page 8 -9 of your Solutions Notes. Practice should be completed for tomorrow.