Hyponatremia with Intravenous SulfamethoxazoleTrimethoprim Katy Stephens Pharm D








- Slides: 19

Hyponatremia with Intravenous Sulfamethoxazole/Trimethoprim Katy Stephens, Pharm. D. PGY 2 Pediatric Pharmacy Resident University of Oklahoma College of Pharmacy Mentor: Pete N. Johnson, Pharm. D. , BCPS, BCPPS, FPPA, FCCM, FASHP Professor, University of Oklahoma College of Pharmacy Oklahoma Society of Health System Pharmacists Spring Meeting April 24, 2021

Disclosures ▪ ▪ I have no known financial disclosures related to the content of this presentation Dr. Pete Johnson has no known financial disclosures related to the content of this presentation For time limitation purposes, sulfamethoxazole/trimethoprim will be verbally referred to as Bactrim throughout this presentation For space limitation purposes, sulfamethoxazole/trimethoprim will be written as SMX/TMP in this presentation 2

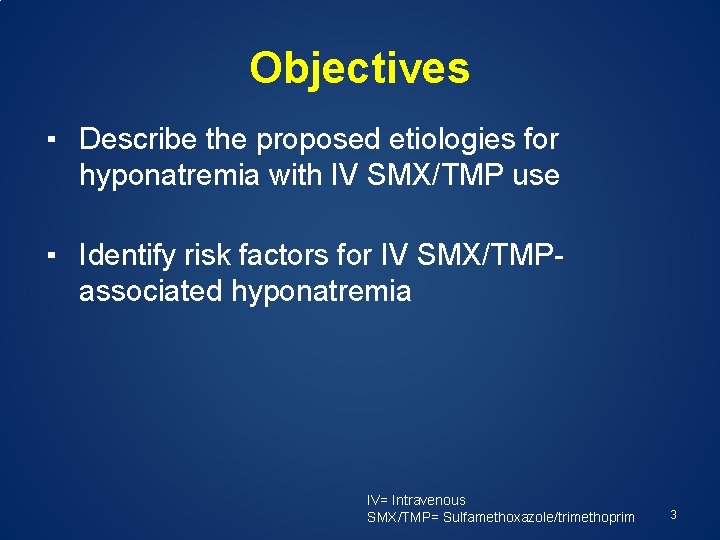
Objectives ▪ Describe the proposed etiologies for hyponatremia with IV SMX/TMP use ▪ Identify risk factors for IV SMX/TMPassociated hyponatremia IV= Intravenous SMX/TMP= Sulfamethoxazole/trimethoprim 3

1. Background

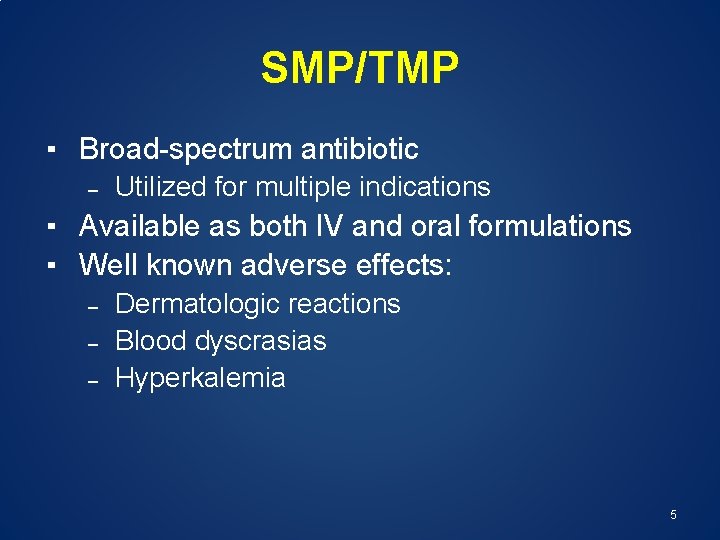
SMP/TMP ▪ Broad-spectrum antibiotic – Utilized for multiple indications ▪ Available as both IV and oral formulations ▪ Well known adverse effects: – – – Dermatologic reactions Blood dyscrasias Hyperkalemia 5

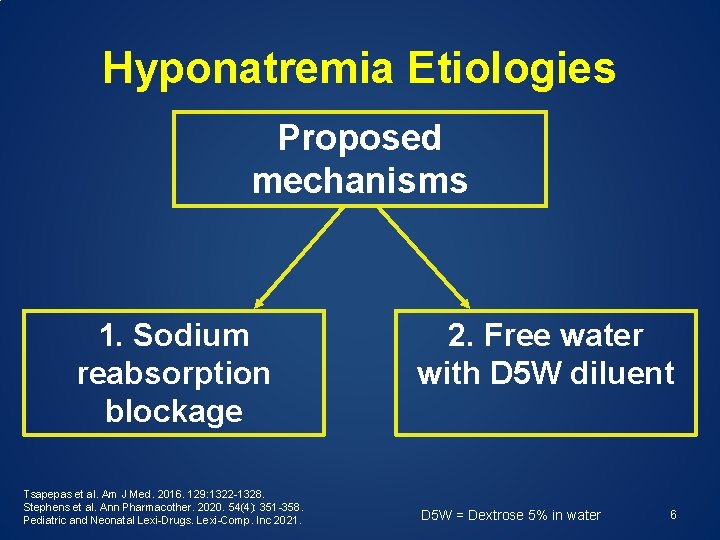
Hyponatremia Etiologies Proposed mechanisms 1. Sodium reabsorption blockage Tsapepas et al. Am J Med. 2016. 129: 1322 -1328. Stephens et al. Ann Pharmacother. 2020. 54(4): 351 -358. Pediatric and Neonatal Lexi-Drugs. Lexi-Comp. Inc 2021. 2. Free water with D 5 W diluent D 5 W = Dextrose 5% in water 6

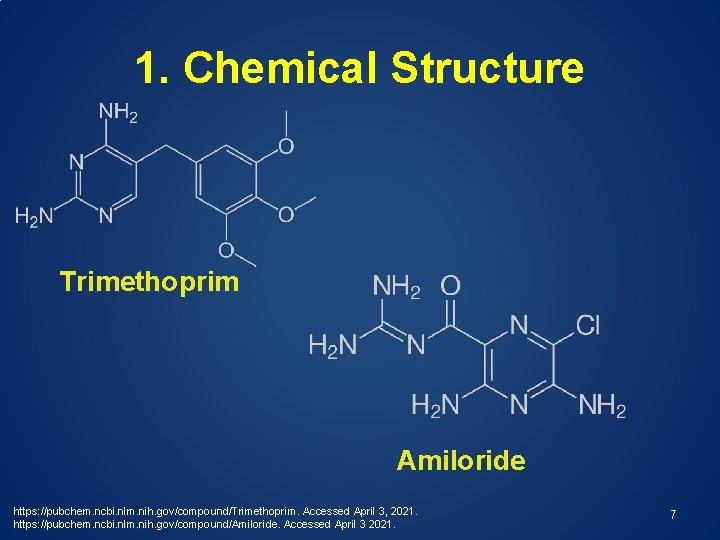
1. Chemical Structure Trimethoprim Amiloride https: //pubchem. ncbi. nlm. nih. gov/compound/Trimethoprim. Accessed April 3, 2021. https: //pubchem. ncbi. nlm. nih. gov/compound/Amiloride. Accessed April 3 2021. 7

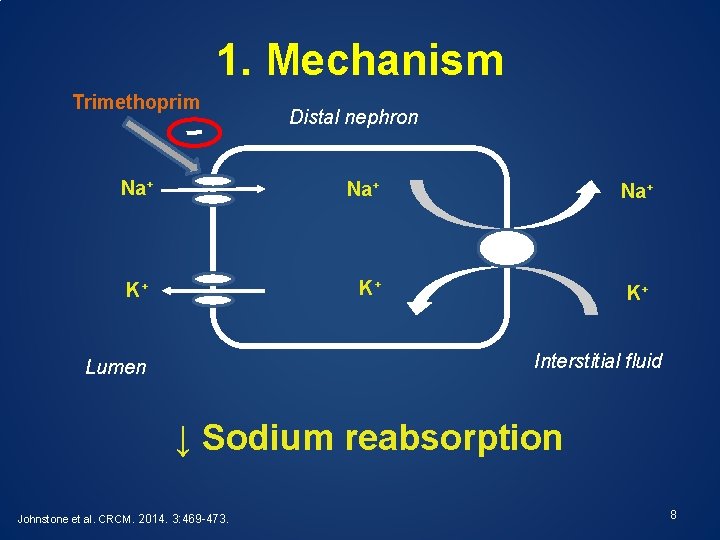
1. Mechanism Trimethoprim Distal nephron Na+ Na+ K+ K+ K+ Interstitial fluid Lumen ↓ Sodium reabsorption Johnstone et al. CRCM. 2014. 3: 469 -473. 8

2. IV Stability ▪ Limited IV stability ▪ Most sources recommend D 5 W for dilution ▪ SMX/TMP available as an 80 -16 mg/m. L IV solution Optimal dilution: 1: 25 ratio of drug: D 5 W 5 m. L of drug diluted in 125 m. L of D 5 W Fluid-restricted dilution: 1: 15 ratio of drug: D 5 W 5 m. L of drug diluted in 75 m. L of D 5 W Pediatric and Neonatal Lexi-Drugs. Lexi-Comp. Inc 2021. 9

2. IV Dilution ▪ Dosing for severe infections is 15 -20 mg/kg/day of TMP component ▪ For optimal stability dilution, patients can receive up to 23 -31 m. L/kg/day of fluid ▪ Concern that this amount of free water can lead to hyponatremia Pediatric and Neonatal Lexi-Drugs. Lexi-Comp. Inc 2021. 10

2. Literature

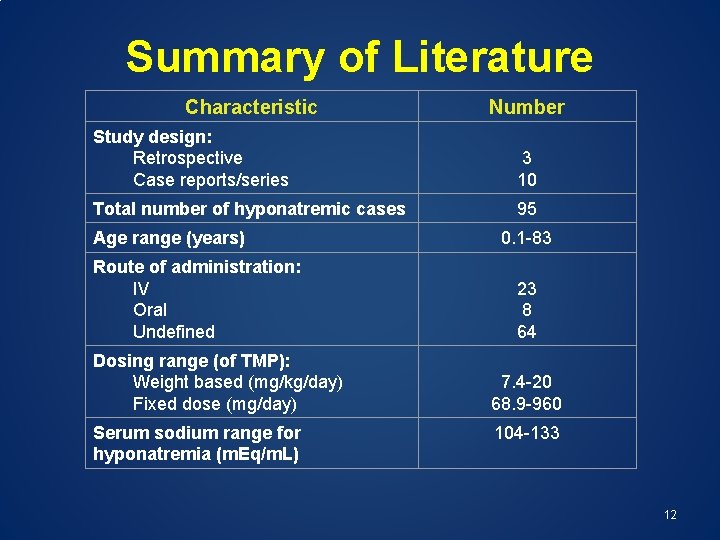
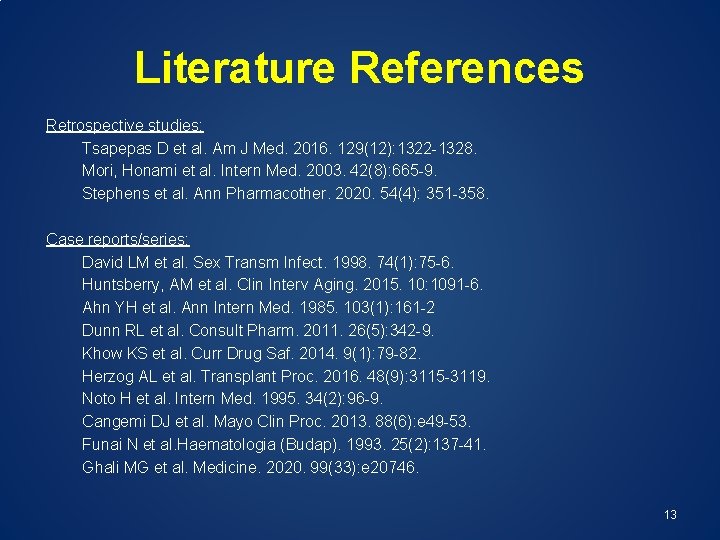
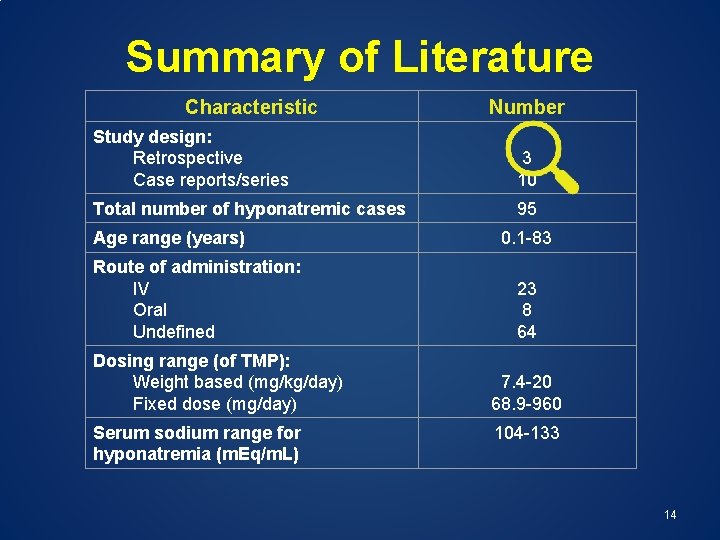
Summary of Literature Characteristic Number Study design: Retrospective Case reports/series 3 10 Total number of hyponatremic cases 95 Age range (years) Route of administration: IV Oral Undefined Dosing range (of TMP): Weight based (mg/kg/day) Fixed dose (mg/day) Serum sodium range for hyponatremia (m. Eq/m. L) 0. 1 -83 23 8 64 7. 4 -20 68. 9 -960 104 -133 12

Literature References Retrospective studies: Tsapepas D et al. Am J Med. 2016. 129(12): 1322 -1328. Mori, Honami et al. Intern Med. 2003. 42(8): 665 -9. Stephens et al. Ann Pharmacother. 2020. 54(4): 351 -358. Case reports/series: David LM et al. Sex Transm Infect. 1998. 74(1): 75 -6. Huntsberry, AM et al. Clin Interv Aging. 2015. 10: 1091 -6. Ahn YH et al. Ann Intern Med. 1985. 103(1): 161 -2 Dunn RL et al. Consult Pharm. 2011. 26(5): 342 -9. Khow KS et al. Curr Drug Saf. 2014. 9(1): 79 -82. Herzog AL et al. Transplant Proc. 2016. 48(9): 3115 -3119. Noto H et al. Intern Med. 1995. 34(2): 96 -9. Cangemi DJ et al. Mayo Clin Proc. 2013. 88(6): e 49 -53. Funai N et al. Haematologia (Budap). 1993. 25(2): 137 -41. Ghali MG et al. Medicine. 2020. 99(33): e 20746. 13

Summary of Literature Characteristic Number Study design: Retrospective Case reports/series 3 10 Total number of hyponatremic cases 95 Age range (years) Route of administration: IV Oral Undefined Dosing range (of TMP): Weight based (mg/kg/day) Fixed dose (mg/day) Serum sodium range for hyponatremia (m. Eq/m. L) 0. 1 -83 23 8 64 7. 4 -20 68. 9 -960 104 -133 14

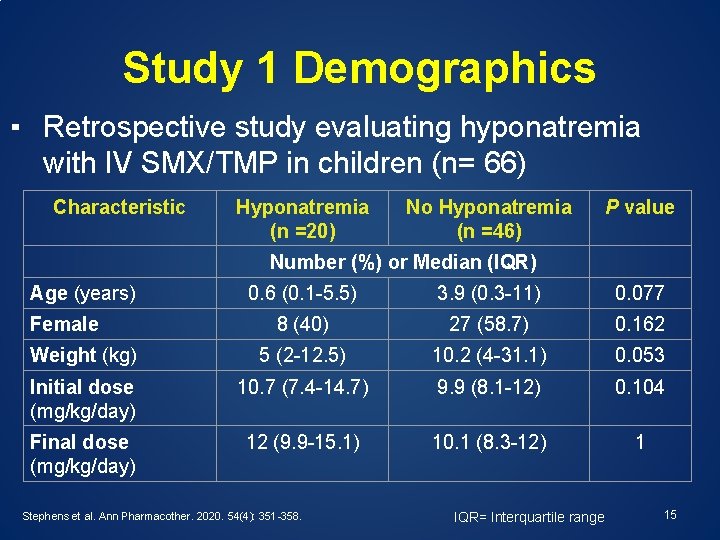
Study 1 Demographics ▪ Retrospective study evaluating hyponatremia with IV SMX/TMP in children (n= 66) Characteristic Hyponatremia (n =20) No Hyponatremia (n =46) P value Number (%) or Median (IQR) Age (years) 0. 6 (0. 1 -5. 5) 3. 9 (0. 3 -11) 0. 077 8 (40) 27 (58. 7) 0. 162 Weight (kg) 5 (2 -12. 5) 10. 2 (4 -31. 1) 0. 053 Initial dose (mg/kg/day) 10. 7 (7. 4 -14. 7) 9. 9 (8. 1 -12) 0. 104 Final dose (mg/kg/day) 12 (9. 9 -15. 1) 10. 1 (8. 3 -12) 1 Female Stephens et al. Ann Pharmacother. 2020. 54(4): 351 -358. IQR= Interquartile range 15

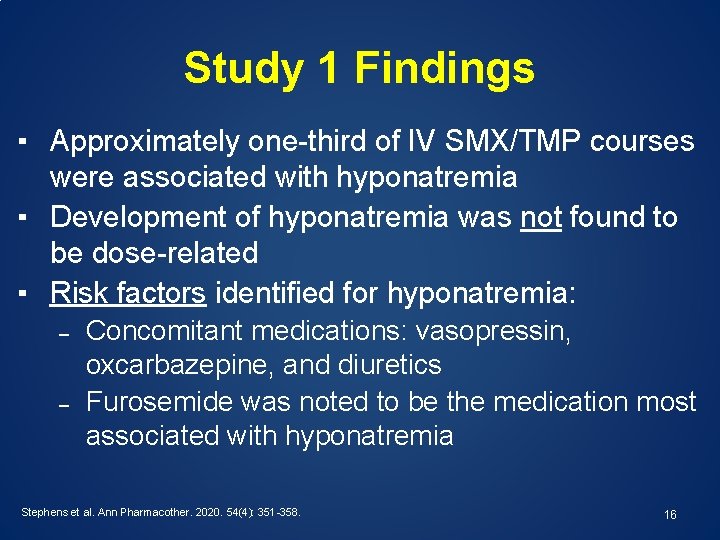
Study 1 Findings ▪ Approximately one-third of IV SMX/TMP courses were associated with hyponatremia ▪ Development of hyponatremia was not found to be dose-related ▪ Risk factors identified for hyponatremia: – – Concomitant medications: vasopressin, oxcarbazepine, and diuretics Furosemide was noted to be the medication most associated with hyponatremia Stephens et al. Ann Pharmacother. 2020. 54(4): 351 -358. 16

3. Clinical Pearls

Clinical Pearls ▪ Hyponatremia is associated with use of IV SMX/TMP ▪ Two proposed etiologies include: – – TMP blocks sodium reabsorption Free water administration due to stability ▪ Risk factors include concomitant use of certain medications and diuretics ▪ Serum sodium should be carefully monitored in patients while on IV SMX/TMP 18

Hyponatremia with Intravenous Sulfamethoxazole/Trimethoprim Katy Stephens, Pharm. D. PGY 2 Pediatric Pharmacy Resident University of Oklahoma College of Pharmacy Mentor: Pete N. Johnson, Pharm. D. , BCPS, BCPPS, FPPA, FCCM, FASHP Professor, University of Oklahoma College of Pharmacy Oklahoma Society of Health System Pharmacists Spring Meeting April 24, 2021
 Line & catheter label - intravenous burette
Line & catheter label - intravenous burette Concepts of medication administration pretest
Concepts of medication administration pretest Cannula size
Cannula size Otorhinolaryngology component word parts
Otorhinolaryngology component word parts Care of iv cannula site ppt
Care of iv cannula site ppt Iv cannula labelling
Iv cannula labelling Complications of iv infusion
Complications of iv infusion Iv cannula labelling
Iv cannula labelling Intravenous medication administration pretest
Intravenous medication administration pretest Hyponatremia causes
Hyponatremia causes Hdl level
Hdl level Severe hyponatremia treatment
Severe hyponatremia treatment Potassium correction formula
Potassium correction formula 2na glucose 18 bun 2.8
2na glucose 18 bun 2.8 Serum osmolarity formula
Serum osmolarity formula Management of hyponatremia
Management of hyponatremia Water deficit formula
Water deficit formula Siadh vs csw
Siadh vs csw Pharm degree
Pharm degree Pharm
Pharm